15 Easy Science Experiments That Teach Kids More Than a Classroom Lesson
Hands-on science experiments ignite children’s curiosity and foster critical thinking skills beyond traditional classroom lessons. By engaging in simple, interactive activities, children connect complex scientific concepts to real-world phenomena, making learning both meaningful and enjoyable. This approach not only enhances their understanding but also cultivates a lifelong passion for discovery and learning.
1. Rainbow in a Jar

Creating a vibrant rainbow in a jar involves layering liquids of varying densities and colors. By carefully pouring substances like honey, dish soap, water, vegetable oil, and rubbing alcohol, each liquid forms a distinct layer based on its density. This experiment illustrates the principles of density and immiscibility, demonstrating how substances with different densities interact. Understanding these concepts is crucial in real-life scenarios, such as oil spills, where oil floats on water due to its lower density, and in natural water stratification, where denser waters settle beneath lighter ones.
2. Dancing Raisins

In the “Dancing Raisins” experiment, raisins are dropped into a glass of clear, carbonated soda. Initially, the raisins sink due to their higher density compared to the soda. As carbon dioxide bubbles from the soda adhere to the rough surfaces of the raisins, they increase the raisins’ buoyancy, causing them to rise. Upon reaching the surface, the bubbles burst, and the raisins sink again, creating a dancing effect. This phenomenon demonstrates principles of buoyancy and gas release, similar to how submarines adjust their buoyancy by controlling ballast tanks to ascend or descend in water. Additionally, it mirrors the behavior of natural gas bubbles in lakes, where gas pockets rise to the surface, affecting water dynamics.
3. Homemade Lava Lamp

Creating a homemade lava lamp involves layering water, oil, food coloring, and an effervescent tablet. When the tablet dissolves in water, it releases carbon dioxide gas, forming bubbles that carry colored water droplets upward through the oil. This process demonstrates the principles of immiscibility and molecular polarity, as water (a polar molecule) and oil (a nonpolar molecule) do not mix. The experiment also illustrates phase separation, akin to how oil slicks float on water due to differences in density and polarity.
4. Invisible Ink Messages

Writing secret messages with lemon juice reveals the chemical reaction behind the ink’s color change. Lemon juice contains carbon compounds that are colorless at room temperature. When exposed to heat, these compounds break down and oxidize, turning brown and making the message visible. This process, known as oxidation, was historically used in espionage, such as during the American Revolutionary War, to conceal communications. Additionally, natural pH indicators like litmus paper change color in response to acidity or alkalinity, demonstrating how chemical reactions can alter the appearance of substances.
5. Balloon-Powered Car

Constructing a simple car propelled by the force of air released from a balloon demonstrates Newton’s third law of motion: for every action, there is an equal and opposite reaction. As the air escapes the balloon, it pushes the car forward. This principle is fundamental in real-world vehicles, where engines expel gases to generate thrust. Additionally, the experiment highlights concepts of propulsion and mass, illustrating how varying the amount of air in the balloon affects the car’s speed and distance traveled.
6. Growing Crystals at Home

Growing crystals at home involves dissolving salts like table salt or Epsom salt in water to create a supersaturated solution. As the water evaporates, the dissolved particles come together to form solid crystals. This process demonstrates solubility, evaporation, and crystal formation. The resulting crystals can be compared to natural formations found in caves, geodes, or snowflakes, where similar processes occur over time.
7. Walking Water

The “Walking Water” experiment demonstrates capillary action, where colored water travels up paper towels from one cup to another, mixing and forming new colors. This process occurs because water molecules are attracted to the cellulose fibers in the paper towel (adhesion) and to each other (cohesion), allowing the water to move against gravity. This principle is similar to how water moves through plants, from roots to leaves, and explains the spreading of marker ink on wet paper.
8. Simple Circuit with a Battery and Light Bulb
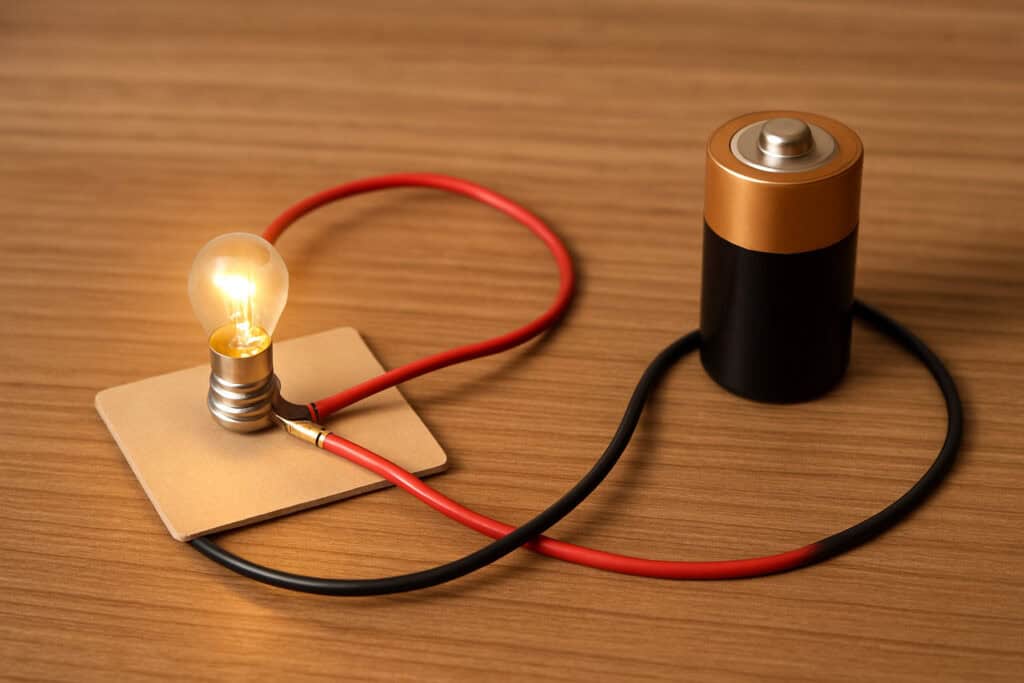
Constructing a simple circuit by connecting a battery, wires, and a light bulb demonstrates the fundamental principles of electricity. When the circuit is complete, electric current flows from the battery’s positive terminal through the wire to the light bulb, causing it to illuminate. This setup introduces basic concepts such as conductors and insulators. Materials like copper are good conductors, allowing electricity to flow easily, while materials like plastic are insulators, preventing the flow of electricity. Understanding these principles is essential, as they are foundational to the operation of household wiring and devices like flashlights.
9. Oobleck: The Non-Newtonian Fluid

Oobleck is a mixture of cornstarch and water that exhibits unique properties, behaving as both a solid and a liquid depending on the applied stress. When pressure is applied quickly, such as striking or squeezing, the mixture becomes rigid, acting like a solid. Conversely, gentle pressure allows it to flow like a liquid. This behavior is due to the shear-thickening nature of non-Newtonian fluids, where viscosity increases with applied stress. Other examples include quicksand, which becomes more liquid-like under stress, and ketchup, which flows more easily when the bottle is tapped.
10. Static Electricity Butterfly

The “Static Electricity Butterfly” experiment demonstrates how rubbing a balloon against your hair and holding it near a tissue-paper butterfly causes the wings to move due to static charge. When you rub the balloon on your hair, electrons transfer from your hair to the balloon, giving the balloon a negative charge and leaving your hair positively charged. Bringing the negatively charged balloon close to the neutral tissue paper induces a positive charge on the near side of the paper, causing the wings to be attracted to the balloon. This experiment illustrates the principles of electron transfer, attraction, and repulsion, similar to how lightning occurs when static electricity builds up in clouds.
11. Egg in a Bottle

The “Egg in a Bottle” experiment demonstrates how changes in air pressure can cause a peeled hard-boiled egg to be sucked into a bottle. When a piece of paper is ignited and placed inside the bottle, the air inside heats up and expands, some escaping through the neck. Once the flame extinguishes, the air cools and contracts, lowering the internal pressure. The higher external air pressure then pushes the egg into the bottle. This illustrates gas laws and pressure differences, similar to how weather systems operate and how suction cups adhere to surfaces.
12. Homemade Volcano

Creating a model volcano using baking soda and vinegar demonstrates a chemical reaction that produces carbon dioxide gas, resulting in a foamy eruption. This experiment illustrates acid-base interactions, where acetic acid (vinegar) reacts with sodium bicarbonate (baking soda) to form carbonic acid, which decomposes into water and carbon dioxide gas. This reaction is similar to real volcanic eruptions, where magma releases dissolved gases, leading to explosive activity.
13. Sun Prints with Construction Paper

Creating sun prints with construction paper involves placing objects on colored paper and exposing them to sunlight, resulting in shadow patterns where the paper remains vibrant, and the areas under the objects fade. This demonstrates how sunlight, particularly ultraviolet (UV) light, can fade dyes through photodegradation, where UV radiation breaks down the chemical bonds in colorants, leading to color loss. This process is similar to how UV light causes sunburn by damaging skin cells and is also utilized in photography, where light-sensitive materials capture images upon exposure to light.
14. Water Cycle in a Bag

Creating a “Water Cycle in a Bag” model allows children to observe the stages of the water cycle—evaporation, condensation, and precipitation—using simple materials.To begin, draw representations of the sun, clouds, and water bodies on a resealable plastic bag. Fill the bag with a small amount of water, add a few drops of blue food coloring if desired, seal it, and tape it to a sunny window. Over time, observe how the water evaporates, condenses on the bag’s surface, and eventually precipitates back into the water at the bottom, simulating the natural water cycle.
15. Magnetic Slime

Creating magnetic slime involves mixing iron oxide powder into a slime base, resulting in a substance that responds to magnetic fields. This experiment demonstrates magnetism, attraction, and physical changes, providing insight into the properties of materials and their interactions with magnetic fields. The iron oxide particles within the slime are attracted to magnets, causing the slime to move or stretch toward the magnetic source. This phenomenon is similar to how magnetic materials are utilized in electronics and magnetic storage devices, where magnetic fields are used to store and retrieve data.
Conclusion

Engaging in hands-on science experiments transforms complex scientific principles into interactive and memorable learning experiences. These activities not only foster curiosity and critical thinking but also enhance understanding through direct exploration. By actively participating in these experiments, children develop a deeper appreciation for the natural world, encouraging a lifelong passion for discovery and learning. Such experiences lay the foundation for continuous curiosity and inquiry beyond the initial experiments.
.article-content-img img { width: 100% }