The Gut Bacteria No One Talks About — But It Controls Your Weight, Mood, and Skin
In 2022, obesity rates soared to 41.9% in the US and 28% in the UK, while depression affected nearly 8.3% of American adults and 17% of UK adults (CDC, NHS). Mounting research implicates the gut microbiome—a complex ecosystem influencing digestion, immunity, and even neurological health—as a pivotal but often overlooked factor in these trends. Sadly, a lack of awareness and late interventions mean gut health remains under-recognized, delaying effective solutions for weight, mood, and skin issues.
1. Akkermansia muciniphila
Akkermansia muciniphila is a lesser-known but highly influential gut bacterium, making up just 3-5% of our total gut microbes. Its primary function is to maintain and strengthen the gut lining by feeding on mucin, a key component of the protective mucus layer. This process helps preserve gut barrier integrity, reducing inflammation and the risk of “leaky gut” syndrome. Studies have shown that higher levels of Akkermansia muciniphila are associated with lower rates of obesity, as this bacterium plays a role in regulating metabolism and reducing fat storage (NIH).
One practical way to support the growth of Akkermansia muciniphila is by increasing dietary fiber, particularly prebiotic fibers found in foods like oats, onions, and asparagus. Fiber acts as fuel for beneficial bacteria, encouraging the proliferation of Akkermansia and other helpful microbes. Research suggests that diets high in fiber can significantly boost levels of this bacterium, supporting both gut health and weight management (Frontiers in Microbiology). Fostering a robust population of Akkermansia muciniphila may be a key strategy for preventing metabolic disorders and supporting overall wellness.
2. Faecalibacterium prausnitzii
Faecalibacterium prausnitzii is one of the most abundant and beneficial bacteria in a healthy human gut, renowned for its strong anti-inflammatory properties. This bacterium produces butyrate, a short-chain fatty acid that nourishes colon cells, reinforces the intestinal barrier, and suppresses inflammatory responses. Notably, Faecalibacterium prausnitzii levels are found to be significantly reduced in individuals with inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis, highlighting its protective role within the gut ecosystem (NIH).
Supporting the growth of Faecalibacterium prausnitzii involves dietary choices rich in soluble fiber and resistant starch, which this bacterium uses to produce beneficial metabolites. Foods such as bananas, oats, barley, and legumes are excellent sources of these prebiotics. Additionally, increasing intake of polyphenol-rich foods like berries, apples, and green tea may further encourage its presence. By focusing on a fiber-rich, plant-based diet, individuals can help cultivate a thriving population of Faecalibacterium prausnitzii, potentially reducing inflammation and supporting long-term gut health (Frontiers in Microbiology).
3. Bacteroides thetaiotaomicron
Bacteroides thetaiotaomicron is a highly adaptable gut bacterium celebrated for its exceptional ability to break down complex carbohydrates. This microbe produces specialized enzymes that digest plant polysaccharides and dietary fibers that would otherwise be indigestible by humans. By converting these carbohydrates into short-chain fatty acids (SCFAs), Bacteroides thetaiotaomicron not only provides energy to the host but also supports a healthy gut lining and influences metabolic health (NIH).
This bacterium’s carbohydrate metabolism is crucial for weight management. Efficient breakdown and fermentation of dietary fiber can help regulate blood sugar, enhance satiety, and reduce fat accumulation. A balanced population of Bacteroides thetaiotaomicron is associated with a healthier body weight and better metabolic profiles, as shown in several human and animal studies (Nature).
Resistant starches—found in foods like cooked-and-cooled potatoes, green bananas, and lentils—are excellent fuel for Bacteroides thetaiotaomicron. Including these foods in the diet can help nourish this bacterium, promote SCFA production, and support both digestive and metabolic health for more effective weight management.
4. Christensenella minuta
Christensenella minuta is a relatively recent discovery in the world of gut microbiota, but it has quickly attracted attention due to its strong association with lean body types. Research has shown that individuals with higher levels of Christensenella minuta tend to have lower body mass indexes (BMIs) compared to those with reduced levels of this bacterium (Nature). Groundbreaking transplantation experiments in mice further underscore its role: when gut bacteria from lean humans rich in Christensenella were transplanted into germ-free mice, the animals remained leaner than those receiving bacteria from obese individuals.
Although the mechanisms are still being explored, Christensenella minuta appears to influence energy metabolism and fat storage, possibly by interacting with other beneficial bacteria and producing metabolites that regulate weight. To foster its growth, experts suggest adopting a diet rich in diverse fibers, especially those from whole grains, fruits, and vegetables. Avoiding excessive processed foods and sugars is also crucial, as these can disrupt the delicate microbial balance. Additionally, consuming fermented foods and maintaining a healthy, active lifestyle may create an environment favorable for Christensenella minuta to thrive, supporting healthy weight and metabolic outcomes (NIH).
5. Roseburia hominis
Roseburia hominis is an important gut bacterium known for its ability to produce butyrate, a short-chain fatty acid that plays a vital role in protecting and nourishing the gut lining. Butyrate serves as the primary energy source for colon cells, helping to maintain the integrity of the intestinal barrier and reduce inflammation. Low levels of Roseburia hominis and butyrate production have been linked to digestive disorders, including irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (NIH).
Beyond gut health, butyrate also has a significant impact on the brain and mood regulation. Research suggests that butyrate can influence the gut-brain axis by modulating neurotransmitter activity and reducing systemic inflammation, potentially lowering the risk of anxiety and depression (Frontiers in Cellular Neuroscience).
To naturally boost butyrate production and support Roseburia hominis, focus on consuming prebiotic-rich foods such as onions, leeks, garlic, and whole grains. Resistant starches—like those found in cooled potatoes and legumes—also feed butyrate-producing bacteria. Including a variety of fiber sources helps ensure a thriving population of these beneficial microbes, benefiting both gut and mental health.
6. Eubacterium hallii
Eubacterium hallii is a key member of the gut microbiome, notable for its ability to engage in “cross-feeding” relationships with other beneficial bacteria. This process involves Eubacterium hallii utilizing metabolic byproducts from neighboring microbes, such as lactate and acetate, and converting them into butyrate—a fatty acid crucial for gut health (NIH). This metabolic cooperation not only enhances butyrate levels but also sustains a balanced microbial ecosystem in the gut.
Recent research has linked the abundance of Eubacterium hallii to improvements in skin health, particularly in reducing skin inflammation and atopic dermatitis symptoms. The anti-inflammatory effects of butyrate and other metabolites produced by this bacterium can modulate immune responses both in the gut and beyond, influencing skin barrier function and reducing flare-ups related to inflammatory skin conditions (Frontiers in Microbiology).
Supporting the growth of Eubacterium hallii involves a diet rich in prebiotics, such as inulin, fructooligosaccharides, and resistant starches found in foods like chicory root, garlic, bananas, and whole grains. These fibers fuel both Eubacterium hallii and its microbial partners, fostering an environment that promotes skin and gut health.
7. Lactobacillus reuteri
Lactobacillus reuteri is a probiotic bacterium that stands out for its profound effects on emotional well-being and social bonding. Research has shown that L. reuteri can stimulate the production of oxytocin, often referred to as the “bonding hormone,” which is involved in trust, empathy, and stress reduction (NIH). Animal studies have demonstrated that supplementation with L. reuteri increases circulating oxytocin levels, resulting in improved social behaviors and enhanced feelings of connectedness.
Clinical trials in humans have highlighted the benefits of Lactobacillus reuteri for stress resilience. In one notable study, individuals consuming L. reuteri exhibited lower levels of the stress hormone cortisol and reported improved mood and coping abilities during challenging situations (PubMed). These findings suggest that this bacterium not only supports gut health but also positively influences the gut-brain axis.
Lactobacillus reuteri is naturally found in various fermented foods, such as kefir, sauerkraut, and certain yogurts. Regular consumption of these foods, or targeted probiotic supplements, can help increase its presence in the gut, potentially enhancing emotional well-being and resilience to stress.
8. Bifidobacterium adolescentis
Bifidobacterium adolescentis is a crucial member of the gut microbiome, particularly valued for its ability to efficiently break down plant fibers and complex carbohydrates. This activity not only aids in digestion but also results in the production of beneficial short-chain fatty acids, which help support gut barrier integrity and overall digestive health (NIH).
Emerging research suggests an intriguing connection between Bifidobacterium adolescentis and mental health—especially in younger populations. Studies indicate that adolescents with higher levels of this bacterium in their gut tend to experience fewer symptoms of anxiety and depression. The proposed mechanism involves the modulation of inflammation and the production of neuroactive compounds that can influence brain function and mood (Psychiatry Research).
To boost Bifidobacterium adolescentis levels, individuals can increase their intake of high-fiber foods such as whole grains, beans, and leafy greens. Probiotic supplements specifically containing B. adolescentis are also available (PubMed). Incorporating these strategies may support both digestive and mental well-being, particularly in adolescents navigating periods of stress or emotional challenge.
9. Parabacteroides distasonis
Parabacteroides distasonis plays a unique and promising role in the gut microbiome through its ability to metabolize bile acids. This process can influence the digestion and absorption of dietary fats as well as regulate cholesterol levels, making Parabacteroides distasonis especially relevant for individuals consuming high-fat diets (NIH).
Animal studies have demonstrated that supplementation with Parabacteroides distasonis can prevent weight gain and reduce fat accumulation, even when mice are fed a high-fat diet (Nature Communications). These effects are believed to result from the bacterium’s modulation of bile acid profiles, which in turn affect metabolic pathways involved in energy expenditure and fat storage. Additionally, Parabacteroides distasonis produces short-chain fatty acids and other metabolites that help regulate inflammation and support metabolic health.
For those following diets rich in fats, promoting the growth of Parabacteroides distasonis may be beneficial. This can be encouraged by consuming a diverse plant-based diet with adequate fiber, as well as considering prebiotic supplementation. Research on this bacterium is ongoing, but its potential in weight management and metabolic regulation is increasingly recognized.
10. Clostridium butyricum

Clostridium butyricum is a powerful butyrate-producing bacterium with a long history of use in functional foods and probiotic supplements, especially across Asia. Butyrate is a short-chain fatty acid that plays a critical role in maintaining gut health, supporting the intestinal barrier, and reducing systemic inflammation. Clostridium butyricum efficiently ferments dietary fibers to produce butyrate, which not only nourishes colon cells but also exerts anti-inflammatory and immune-modulating effects (NIH).
This bacterium is widely utilized in Japanese and Chinese probiotics for its safety and broad health benefits. It has been shown to help restore healthy gut flora after antibiotic use and protect against gastrointestinal disorders. Recent research also highlights its potential in reducing symptoms of eczema, particularly in children. Clinical studies suggest that Clostridium butyricum supplementation can decrease the severity of atopic dermatitis by modulating immune responses and improving the composition of the gut microbiome (Frontiers in Microbiology).
To support Clostridium butyricum, include a variety of prebiotic fibers in your diet and consider Asian probiotic products containing this beneficial bacterium for targeted gut and skin health support.
11. Blautia wexlerae
Blautia wexlerae is a relatively recent addition to the list of notable gut bacteria, but its importance in metabolic health is quickly gaining attention. Studies have found that higher levels of Blautia wexlerae are associated with lower body mass index (BMI) and a reduced risk of obesity and metabolic syndrome (Nature Communications). This bacterium is believed to influence energy metabolism by producing short-chain fatty acids, such as acetate, which help regulate appetite, fat storage, and inflammation.
Researchers are investigating the mechanisms by which Blautia wexlerae supports a healthy weight. Early evidence suggests it may interact with other beneficial microbes and contribute to a balanced gut ecosystem, further supporting metabolic function (NIH).
To promote the growth of Blautia wexlerae, a diet high in plant-based fibers appears beneficial. Foods such as whole grains, beans, lentils, and a variety of vegetables provide the prebiotics necessary for its proliferation. Fermented foods, which encourage overall microbiome diversity, may also indirectly support Blautia wexlerae and general gut health, offering another tool for weight management and metabolic wellness.
12. Oscillibacter valericigenes
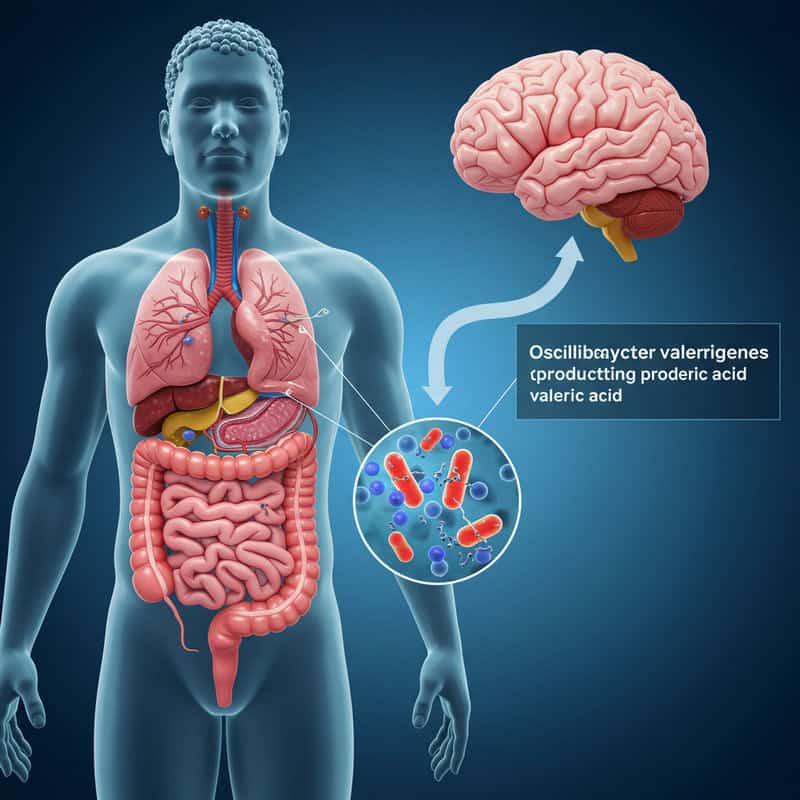
Oscillibacter valericigenes is an emerging player in the gut-brain axis, with growing evidence highlighting its significance in mood regulation and mental health. This bacterium is known for producing valeric acid, a short-chain fatty acid that can interact with the nervous system and potentially influence neurotransmitter activity. Animal studies have demonstrated that higher levels of Oscillibacter valericigenes are linked to reduced anxiety-like behaviors, suggesting a direct connection between this microbe and emotional resilience (Nature).
Research in rodent models indicates that Oscillibacter valericigenes communicates with the brain via gut-brain signaling pathways, modulating stress responses and emotional regulation. Mice with greater abundance of this bacterium exhibit less behavioral despair and improved coping skills under stress (NIH).
However, chronic psychological stress has been shown to reduce the abundance of Oscillibacter valericigenes in the gut, potentially weakening its mood-supportive effects. To help maintain healthy levels, stress management, a diverse fiber-rich diet, and lifestyle interventions that support microbial balance may prove beneficial for both gut and mental wellness.
13. Alistipes putredinis
Alistipes putredinis is a bacterium that has become increasingly prevalent in individuals consuming Western-style diets, which are often high in animal fats, processed foods, and low in fiber. While its precise role is still being unraveled, recent studies have observed that higher levels of Alistipes putredinis may be associated with an increased risk of depression and mood disorders (Nature). The exact mechanisms are not fully understood, but it is believed that changes in gut microbiota composition, influenced by diet, may impact the production of neuroactive metabolites and inflammatory pathways that affect brain health.
Interestingly, some research suggests that Alistipes putredinis may also play a dual role—while its abundance is linked to potential negative mood outcomes, it can contribute to the gut’s ability to ferment proteins and complex carbohydrates, a process more common in high-protein, low-fiber diets (PubMed).
To help maintain a balanced presence of Alistipes putredinis, dietary adjustments such as increasing plant-based fibers, reducing excessive animal fats, and including more whole fruits and vegetables are recommended. A diverse, fiber-rich diet can foster a healthier microbial ecosystem, potentially reducing depression risk linked to gut dysbiosis.
14. Veillonella parvula
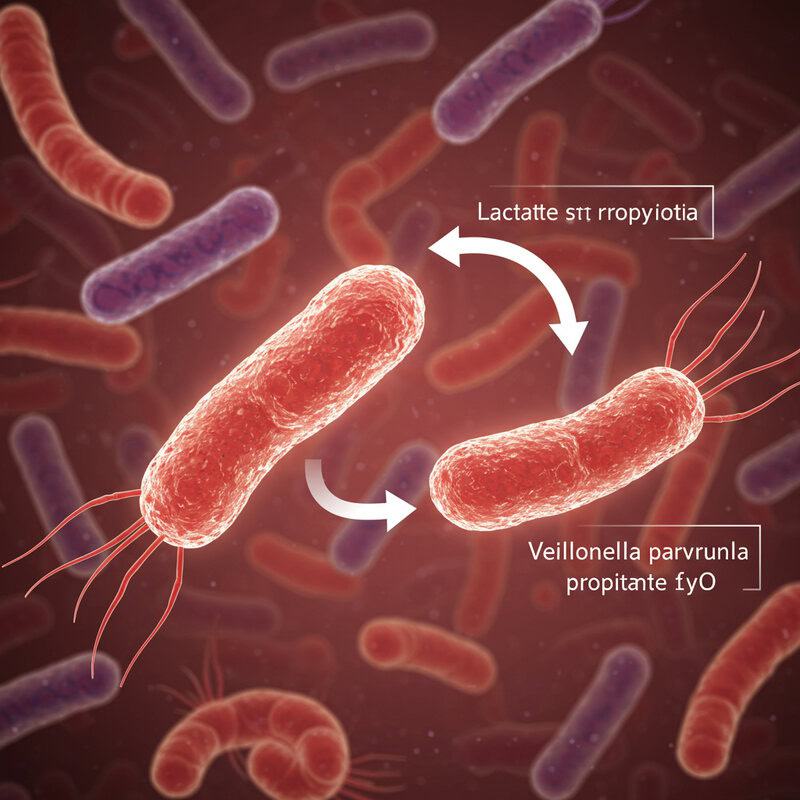
Veillonella parvula is a fascinating gut bacterium recognized for its unique metabolic talent: it converts lactate—a byproduct of muscle exertion—into propionate, a short-chain fatty acid that can be used as an energy source by the body. This process not only helps clear lactate from the bloodstream but also recycles it into a form that supports metabolic health (Nature).
Recent studies have spotlighted Veillonella parvula in the context of athletic performance. Researchers found that endurance athletes, such as marathon runners, tend to have higher levels of this bacterium in their gut after intense exercise. Further, animal models receiving transplants of Veillonella demonstrated enhanced endurance and improved exercise capacity, likely due to the efficient conversion of lactate into usable energy (NIH).
These findings suggest that supporting Veillonella parvula could have meaningful implications for exercise recovery and performance. Consuming a diet rich in complex carbohydrates and plant fibers may help foster a balanced gut environment, encouraging the growth of this performance-boosting microbe and potentially aiding in faster, more efficient recovery from strenuous workouts.
15. Bifidobacterium longum
Bifidobacterium longum is a widely recognized probiotic bacterium with well-documented benefits for digestive and mental health. One of its most notable properties is its anti-stress effect, as demonstrated by multiple clinical studies. Bifidobacterium longum can modulate the gut-brain axis, influencing the body’s response to psychological stress and supporting emotional resilience (Frontiers in Neuroscience).
Double-blind, placebo-controlled trials have shown that individuals taking Bifidobacterium longum supplements report improved mood, reduced anxiety, and better sleep quality compared to those given a placebo. These positive effects are likely due to the bacterium’s ability to reduce systemic inflammation and influence neurotransmitter production in the gut, which in turn impacts brain chemistry and stress levels (NIH).
Bifidobacterium longum is considered safe and is commonly found in fermented foods such as yogurt, kefir, and certain probiotic supplements. Regularly incorporating these foods into the diet, or choosing a reputable probiotic product containing B. longum, can help support both gut and mental health, offering a natural means to bolster stress resilience and emotional well-being.
16. Prevotella copri
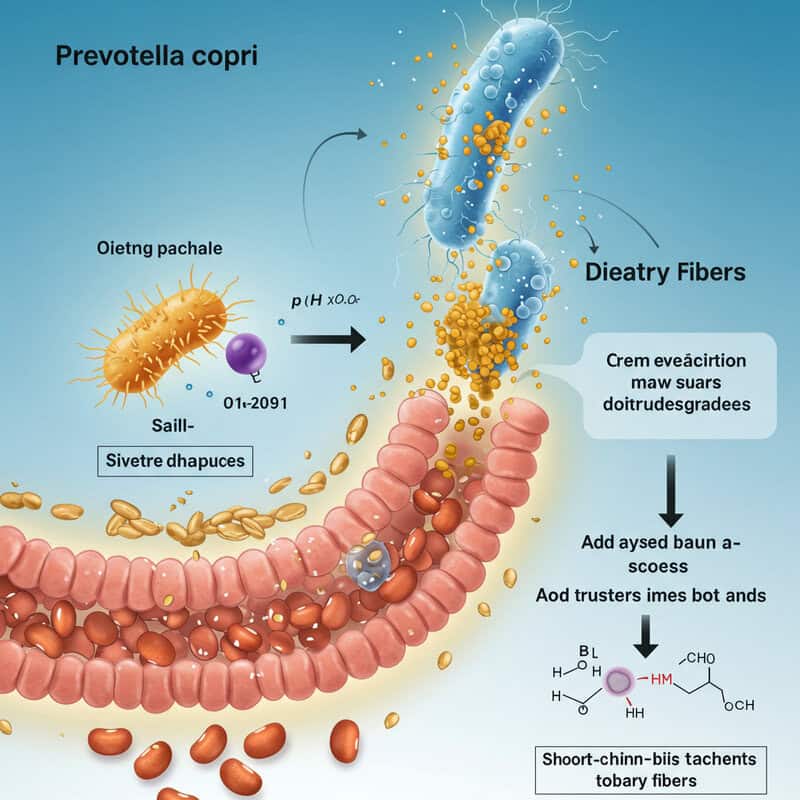
Prevotella copri is a carbohydrate-fermenting bacterium that plays a prominent role in the digestion of dietary fibers, particularly those found in whole grains, legumes, and vegetables. By breaking down these complex carbohydrates, Prevotella copri produces short-chain fatty acids, such as propionate and acetate, which provide energy for colon cells and contribute to anti-inflammatory effects in the gut (Nature).
Interestingly, the abundance of Prevotella copri varies significantly by region and dietary pattern. Populations consuming high-fiber, plant-based diets—common in non-Western countries—tend to have much higher levels of this bacterium compared to those with Western diets rich in animal protein and processed foods (Cell). This difference is thought to contribute to the lower rates of metabolic and inflammatory diseases observed in these regions.
To maximize gut diversity and encourage the growth of Prevotella copri, it is beneficial to consume a wide variety of fiber-rich plant foods. Incorporating whole grains, beans, and fresh produce daily, while minimizing processed foods, can help establish a more diverse and resilient gut microbiome, supporting both digestive and overall health.
17. Ruminococcus bromii
Ruminococcus bromii is a key specialist in the gut microbiome, renowned for its exceptional ability to break down resistant starch—a form of carbohydrate that escapes digestion in the upper gut and reaches the colon intact. This bacterium’s unique enzymatic machinery allows it to efficiently ferment resistant starch into beneficial short-chain fatty acids, particularly butyrate, which support colon health and maintain the integrity of the gut lining (Nature).
The activity of Ruminococcus bromii has important implications for metabolic health, especially in regulating blood sugar. By breaking down resistant starch and facilitating the production of butyrate, this bacterium helps modulate blood glucose levels and improve insulin sensitivity. Research suggests that individuals with higher levels of Ruminococcus bromii may experience better glycemic control, making it relevant for those managing or seeking to prevent type 2 diabetes (NIH).
To support the growth of Ruminococcus bromii, include foods naturally high in resistant starch, such as cooked-and-cooled potatoes, green bananas, legumes, lentils, and whole grains. Regular consumption of these foods not only nourishes this important bacterium but also promotes a healthier, more stable blood sugar profile.
18. Eggerthella lenta
Eggerthella lenta occupies a unique niche in the gut microbiome due to its direct involvement in the metabolism of certain medications, particularly those used to treat heart conditions. This bacterium is capable of transforming specific compounds through enzymatic activity, notably inactivating the cardiac drug digoxin, which is commonly prescribed for heart failure and arrhythmias (NIH). This interaction can significantly alter the effectiveness of the medication, making Eggerthella lenta a critical consideration in personalized medicine.
Researchers have found that the abundance of Eggerthella lenta and its metabolic activity can be influenced by dietary factors, such as the presence of certain amino acids like arginine, which can inhibit the drug-inactivating process (Science). This discovery highlights the complex interplay between diet, gut microbes, and drug efficacy, underscoring the importance of understanding individual microbiome profiles when managing chronic conditions.
If you are taking heart medications—especially digoxin—or other prescription drugs, it is essential to consult your healthcare provider before making significant dietary or probiotic changes. Medical guidance can help ensure optimal drug effectiveness and prevent unintended interactions mediated by the gut microbiome.
19. Coprococcus comes
Coprococcus comes is a gut bacterium that has garnered significant interest due to its connection with the production of happiness-related neurotransmitters. Research shows that Coprococcus comes can promote the synthesis of dopamine, a key neurotransmitter involved in feelings of pleasure, motivation, and emotional well-being (Nature Microbiology). This link between gut microbes and brain chemistry underpins the concept of the gut-brain axis, highlighting the far-reaching influence of the microbiome on mood and mental health.
Large-scale population studies have observed that individuals with a higher abundance of Coprococcus comes in their gut tend to report greater overall happiness and lower rates of depression. These findings suggest that supporting this microbe may be a promising avenue for enhancing resilience and emotional balance (NIH).
Mood-supportive habits that help cultivate Coprococcus comes include consuming a fiber-rich diet—especially with whole grains, fruits, and vegetables—engaging in regular physical activity, managing stress, and maintaining social connections. These behaviors not only boost beneficial gut bacteria but can also positively shape neurotransmitter production and overall emotional health.
20. Methanobrevibacter smithii
Methanobrevibacter smithii is a dominant archaeon in the human gut, best known for its role in producing methane gas. Unlike most bacteria, Methanobrevibacter smithii utilizes hydrogen and carbon dioxide generated by other gut microbes to produce methane, a process that can significantly influence digestive function (Nature Reviews Microbiology).
Elevated levels of this organism have been linked to slower intestinal transit, resulting in symptoms such as constipation, bloating, and abdominal discomfort. Clinical studies have shown that people with higher methane production, as detected in breath tests, are more likely to experience chronic constipation and feelings of fullness (NIH). This is because methane can decrease gut motility, slowing the movement of food through the digestive tract.
Dietary changes can help manage symptoms associated with high Methanobrevibacter smithii levels. Reducing intake of fermentable carbohydrates (such as those found in beans, onions, and certain whole grains) and following a low-FODMAP diet may decrease methane production and alleviate bloating. Consulting a healthcare provider or dietitian can help tailor dietary strategies to individual gut profiles for improved digestive comfort.
21. Desulfovibrio piger
Desulfovibrio piger is a sulfate-reducing bacterium in the gut microbiome, notable for its ability to produce hydrogen sulfide gas during the fermentation of certain sulfur-containing compounds (NIH). While small amounts of hydrogen sulfide can play regulatory roles in gut health, excessive production by Desulfovibrio piger has been linked to negative effects, including irritation of the gut lining and increased intestinal permeability.
Emerging studies have found connections between elevated levels of Desulfovibrio piger and inflammatory skin flare-ups, such as eczema and psoriasis. The hydrogen sulfide gas produced by this bacterium may contribute to systemic inflammation, impacting not only the gut but also the skin and other organs (Journal of Investigative Dermatology).
Recognizing intolerance to excess hydrogen sulfide may involve noting recurring symptoms like bloating, foul-smelling gas, and skin outbreaks after consuming foods rich in sulfur (e.g., eggs, garlic, cruciferous vegetables). If these signs are persistent, it may indicate an overgrowth of Desulfovibrio piger. Consulting with a healthcare provider can help identify and address underlying microbial imbalances for healthier skin and digestion.
22. Bacteroides fragilis
Bacteroides fragilis is a prominent gut bacterium recognized for its powerful role in modulating the immune system. Unlike many other microbes, Bacteroides fragilis produces a unique molecule called polysaccharide A (PSA), which helps balance immune responses and prevent harmful inflammation in the gut and beyond (NIH). This regulatory effect supports the maintenance of immune tolerance, reducing the risk of autoimmune and inflammatory diseases.
Recent studies have explored the impact of Bacteroides fragilis on neurological diseases such as multiple sclerosis (MS). Animal research has shown that PSA from this bacterium can protect against the development and severity of MS-like symptoms by modulating immune cell activity and reducing neuroinflammation (Nature). These findings highlight the far-reaching influence of the gut microbiome on brain and immune health.
Probiotic research is now investigating the therapeutic potential of Bacteroides fragilis strains, particularly those that produce PSA. While commercial probiotics containing this microbe are not yet widely available, ongoing studies continue to explore its safety and efficacy as a next-generation probiotic for immune and neurological disorders.
23. Streptococcus thermophilus
Streptococcus thermophilus is a widely used probiotic bacterium, particularly known for its essential role in the fermentation of yogurt and other cultured dairy products. This lactic acid-producing microbe helps transform milk into yogurt, imparting a creamy texture and characteristic tang, as well as increasing the bioavailability of nutrients like calcium and B vitamins (NIH).
Beyond its digestive benefits, Streptococcus thermophilus also supports skin health. Research suggests that this bacterium can strengthen the skin barrier by enhancing ceramide production, a lipid critical for skin hydration and protection (Journal of Investigative Dermatology). This makes it potentially helpful for individuals experiencing skin dryness or sensitivity, as a healthy skin barrier is key to preventing irritation and flare-ups.
For those seeking to improve digestive function, immune support, or skin barrier health, incorporating cultured dairy products like yogurt or kefir, which contain live Streptococcus thermophilus, may be beneficial. Individuals sensitive to dairy can look for lactose-free options or non-dairy yogurts cultured with this bacterium to enjoy similar health advantages without discomfort.
24. Anaerostipes caccae
Anaerostipes caccae is a beneficial gut bacterium recognized for its robust production of short-chain fatty acids (SCFAs), particularly butyrate and acetate. These SCFAs serve as the primary energy source for colon cells and play a crucial role in maintaining the integrity of the gut lining, reducing inflammation, and supporting overall colon health (NIH).
Butyrate produced by Anaerostipes caccae has been associated with lower risks of colon cancer and inflammatory bowel diseases, as it helps regulate immune responses in the gut and promotes a healthy intestinal barrier. Consistent production of SCFAs also contributes to a more acidic environment in the colon, inhibiting the growth of harmful bacteria and pathogens (Frontiers in Microbiology).
To encourage the growth of Anaerostipes caccae, a diet rich in dietary fibers is essential. Excellent sources of fiber include whole grains, legumes, root vegetables, and fruits such as apples and pears. Regular intake of these plant-based foods not only supports Anaerostipes caccae but also fosters a thriving, balanced gut microbiome for optimal digestive and colon health.
25. Collinsella aerofaciens
Collinsella aerofaciens is a prominent gut bacterium with a specialized role in cholesterol metabolism. This microbe influences the absorption and transformation of cholesterol in the intestines, which in turn can impact blood lipid levels and cardiovascular health (Frontiers in Microbiology). Alterations in the abundance of Collinsella aerofaciens have been observed in individuals with metabolic syndrome, a cluster of conditions including elevated cholesterol, high blood pressure, and insulin resistance.
Research has shown that people with metabolic syndrome or obesity often have reduced levels of Collinsella aerofaciens in their gut compared to healthy individuals (NIH). These changes may contribute to impaired cholesterol handling and increased cardiovascular risk. Furthermore, Collinsella aerofaciens may be involved in producing beneficial metabolites that protect the gut lining and modulate inflammation.
To support a healthy balance of Collinsella aerofaciens, focus on a diet rich in plant-based foods, such as whole grains, legumes, nuts, and vegetables. Reducing intake of saturated fats and highly processed foods while increasing dietary fiber can foster an environment where this bacterium—and overall cholesterol metabolism—can thrive for better metabolic and heart health.
26. Sutterella wadsworthensis
Sutterella wadsworthensis is a gut bacterium that has gained attention due to its increased prevalence in children with autism spectrum disorder (ASD). Several studies have reported higher levels of Sutterella wadsworthensis in the gut microbiota of children diagnosed with ASD compared to neurotypical peers (NIH). While the precise implications of this association are still being investigated, some researchers hypothesize that Sutterella may influence gut-brain communication, possibly contributing to gastrointestinal symptoms and behavioral patterns observed in ASD.
Ongoing research is exploring whether Sutterella wadsworthensis plays a direct role in the development or severity of autism-related symptoms, or if its presence is simply a marker of altered gut ecology. Early findings suggest that changes in the abundance of this bacterium could impact immune regulation and intestinal barrier function, but more studies are needed to clarify these effects (Frontiers in Neuroscience).
If your child has been diagnosed with ASD and you are concerned about gut health or gastrointestinal symptoms, it’s important to discuss your observations with a pediatrician. They can provide guidance on dietary strategies, probiotic options, and the latest research to support your child’s digestive and neurological well-being.
27. Enterococcus faecium
Enterococcus faecium is a gut bacterium that has become notorious in recent decades due to its remarkable capacity for antibiotic resistance. While Enterococcus faecium is a natural inhabitant of the human intestinal tract, certain strains have acquired resistance to multiple antibiotics, including vancomycin, leading to the emergence of vancomycin-resistant enterococci (VRE) (CDC). These resistant strains pose significant challenges in hospital settings, where they can cause difficult-to-treat infections, particularly in immunocompromised patients.
Hospital outbreaks of VRE are a major public health concern, as these bacteria can persist on surfaces and medical equipment, spreading easily between patients. Infections may manifest as urinary tract infections, bloodstream infections, or wound infections, and are associated with increased morbidity and longer hospital stays (NIH).
General hygiene practices are crucial for preventing the spread of Enterococcus faecium in both healthcare and community settings. Regular handwashing with soap and water, thorough cleaning of frequently-touched surfaces, and prudent use of antibiotics are essential strategies. Patients and caregivers should follow infection control guidelines, especially in hospitals, to reduce the risk of resistant bacterial infections.
28. Propionibacterium acnes
Propionibacterium acnes (now also known as Cutibacterium acnes) is a bacterium primarily found on the skin, but its influence extends to the gut-skin axis. P. acnes thrives in oily environments, particularly within sebaceous (oil) glands, where it breaks down sebum and releases inflammatory substances that contribute to acne formation (NIH). This inflammation leads to the redness, swelling, and pustules characteristic of acne outbreaks.
Emerging research suggests a strong connection between gut health and skin conditions like acne. Imbalances in the gut microbiome, including the overgrowth of certain bacteria and increased intestinal permeability, may influence systemic inflammation and immune responses, thereby exacerbating skin problems (Frontiers in Microbiology).
Practical skincare routines to manage Propionibacterium acnes involve gentle cleansing with non-comedogenic products, avoiding harsh scrubbing, and using topical treatments with ingredients like benzoyl peroxide or salicylic acid. Supporting gut health with a balanced, fiber-rich diet and adequate hydration can also help reduce systemic inflammation, providing a holistic approach to acne prevention and skin care.
29. Saccharomyces boulardii
Saccharomyces boulardii is a unique probiotic, as it is not a bacterium but a beneficial yeast. Unlike most probiotics that are bacterial, Saccharomyces boulardii has distinct properties that make it especially effective in preventing and treating various forms of diarrhea, including antibiotic-associated and traveler’s diarrhea (NIH). Its robust cell wall allows it to survive stomach acid and reach the intestines, where it can outcompete harmful pathogens, modulate immune responses, and restore microbial balance.
Clinical trials have shown that Saccharomyces boulardii significantly reduces the risk and duration of diarrhea in both children and adults. It is often recommended alongside antibiotics to help maintain gut function and minimize side effects like gastrointestinal upset (Cochrane Library).
When using Saccharomyces boulardii supplements, it is important to select reputable, high-quality products and follow recommended dosages. It is generally considered safe for most people, but those with weakened immune systems or specific medical conditions should consult a healthcare provider before starting supplementation to ensure safety and effectiveness.
30. Lactococcus lactis
Lactococcus lactis is a lactic acid bacterium renowned for its central role in the fermentation of dairy products, particularly in cheese-making. This bacterium helps ferment milk sugars into lactic acid, which curdles milk proteins and creates the characteristic texture and flavor of cheeses and cultured dairy foods. Lactococcus lactis is widely used in the production of soft cheeses like brie, camembert, and cream cheese, as well as in buttermilk and sour cream (NIH).
Beyond its culinary applications, Lactococcus lactis has been studied for its potential benefits in skin hydration and barrier function. Some research indicates that topically applied or orally ingested L. lactis may enhance skin moisture and reduce symptoms of dryness, possibly by modulating the immune response and influencing the skin-gut axis (Journal of Investigative Dermatology).
Dietary applications of Lactococcus lactis are broad and accessible. Consuming naturally fermented cheeses, cultured buttermilk, and probiotic dairy products can introduce this beneficial bacterium into the gut. For those with lactose intolerance, some fermented dairy products with L. lactis are easier to digest and can support both digestive and skin health.
31. Clostridium ramosum
Clostridium ramosum is a gut bacterium that has drawn attention in recent years due to its association with diet-induced obesity, particularly in the context of high-fat diets. Animal studies have shown that mice colonized with Clostridium ramosum and fed a high-fat diet tend to gain significantly more weight and fat mass than those lacking this bacterium, even when their calorie intake is the same (Nature). The proposed mechanism involves C. ramosum enhancing the absorption of dietary fats and possibly influencing metabolic signaling pathways that promote fat storage.
The findings suggest that an overabundance of Clostridium ramosum in the gut may exacerbate the effects of a diet rich in processed fats, contributing to weight gain and metabolic disturbances. To counteract this risk, nutrition experts recommend limiting the intake of processed and saturated fats, commonly found in packaged snacks, fried foods, and fatty meats. Instead, focus on whole, unprocessed foods and healthy fats from sources like avocados, nuts, seeds, and olive oil. Increasing dietary fiber from vegetables and whole grains may also help balance the gut microbiome and discourage the proliferation of obesity-associated bacteria like Clostridium ramosum.
32. Bifidobacterium bifidum
Bifidobacterium bifidum is a foundational member of the human gut microbiome, present from early infancy and renowned for its immune-enhancing properties. This probiotic bacterium helps maintain a balanced immune response by promoting the development of regulatory T cells, which are crucial in preventing excessive inflammation and allergic reactions (NIH).
Clinical studies have shown that supplementation with Bifidobacterium bifidum can significantly reduce the incidence and severity of allergic conditions, such as eczema and allergic rhinitis, particularly in infants and children. For example, one randomized controlled trial found that infants receiving B. bifidum had fewer atopic symptoms and improved skin health compared to those receiving a placebo (Karger). These benefits are thought to result from the bacterium’s ability to enhance mucosal barrier function and modulate immune signaling.
When selecting probiotic products containing Bifidobacterium bifidum, look for reputable brands that specify the strain and provide a viable count of colony-forming units (CFUs) per serving. Refrigerated or shelf-stable formulations with third-party quality testing are preferable. Regular intake of such probiotics, along with a high-fiber diet, can help support immune resilience and reduce allergy risk.
33. Dorea formicigenerans
Dorea formicigenerans is a gut bacterium that has been increasingly linked to gastrointestinal disorders, most notably irritable bowel syndrome (IBS). Studies indicate that individuals with IBS often exhibit elevated levels of Dorea formicigenerans in their gut microbiome compared to healthy controls (Nature). While the exact mechanisms are still being explored, it is believed that this bacterium may contribute to increased gas production, altered gut motility, and low-grade inflammation—common features of IBS.
Tracking symptoms such as bloating, abdominal pain, irregular bowel habits, and food intolerances can help individuals and healthcare providers identify potential links between gut microbiota imbalances and IBS flares. Keeping a food and symptom diary is a practical approach for recognizing triggers and patterns that correlate with the presence of Dorea formicigenerans or other gut bacteria.
If IBS symptoms are persistent, severe, or significantly impact quality of life, it is important to seek specialist care from a gastroenterologist. Diagnostic tests, dietary interventions (such as low-FODMAP diets), and gut microbiome analysis may help tailor personalized treatment strategies. Early intervention and professional guidance can provide symptom relief and improve long-term digestive health.
34. Escherichia coli Nissle 1917
Escherichia coli Nissle 1917 (EcN) is a unique, non-pathogenic probiotic strain of E. coli distinguished from its harmful relatives by its beneficial effects on gut health. Discovered over a century ago, EcN has been extensively studied for its therapeutic use in managing inflammatory bowel diseases, particularly ulcerative colitis (NIH). Clinical trials have demonstrated that EcN can induce and maintain remission in patients with ulcerative colitis, sometimes offering effectiveness comparable to standard pharmaceutical treatments.
The mechanism behind EcN’s benefits involves modulation of the immune system, strengthening the gut barrier, and outcompeting pathogenic bacteria for resources and attachment sites. Its safety profile is well established, with few reported side effects when used as directed (Gastroenterology).
For those interested in trying Escherichia coli Nissle 1917, it is crucial to obtain the probiotic from reputable sources, such as licensed pharmacies or healthcare providers, as over-the-counter and online products may not always meet quality or regulatory standards. Always consult a healthcare professional before starting EcN, especially if you have a compromised immune system or a history of serious illness.
35. Lactobacillus plantarum
Lactobacillus plantarum is a versatile probiotic bacterium prized for its potent antioxidative properties. By neutralizing free radicals and reducing oxidative stress, Lactobacillus plantarum helps protect cells throughout the body, including those in the skin (NIH). This antioxidative action is particularly valuable in combating the cellular damage that contributes to skin aging, such as wrinkle formation, loss of elasticity, and dullness.
Studies have shown that supplementation or regular consumption of Lactobacillus plantarum can improve skin moisture, enhance barrier function, and even reduce the appearance of fine lines by supporting the skin’s repair mechanisms (Journal of Investigative Dermatology). The bacterium’s anti-inflammatory effects further boost its reputation as a natural ally in maintaining youthful, resilient skin.
Lactobacillus plantarum is abundant in a wide array of fermented vegetables, including sauerkraut, kimchi, pickles, and certain olives. Incorporating these foods into your diet is a flavorful and effective way to introduce this beneficial probiotic, helping to support both gut and skin health while harnessing its anti-aging properties.
36. Clostridium leptum
Clostridium leptum is a key member of the gut microbiome’s Firmicutes group and is highly regarded for its role in maintaining gut health. Research has shown that individuals with Crohn’s disease and other forms of inflammatory bowel disease (IBD) often have a significant reduction in Clostridium leptum populations compared to healthy individuals (NIH). This decrease is associated with impaired production of short-chain fatty acids, especially butyrate, which is essential for nourishing colon cells and regulating inflammation in the gut.
Dietary interventions that increase fiber and prebiotic intake have been found to support the growth and activity of Clostridium leptum. Foods rich in soluble fiber, such as oats, apples, legumes, and root vegetables, as well as prebiotics like inulin and fructooligosaccharides, can help restore beneficial levels of this bacterium (Frontiers in Microbiology).
Monitoring gut symptoms—such as changes in stool consistency, abdominal pain, and bloating—can provide valuable feedback on how dietary changes are influencing the microbiome. If symptoms persist or worsen, consulting with a gastroenterologist or registered dietitian is recommended for targeted testing and personalized dietary guidance to optimize gut health.
37. Bacteroides vulgatus
Bacteroides vulgatus is a common gut bacterium that has been increasingly studied for its association with metabolic syndrome—a cluster of conditions including obesity, high blood pressure, elevated blood sugar, and abnormal cholesterol levels. Recent research has found that an overabundance of Bacteroides vulgatus may contribute to inflammation and metabolic disturbances, particularly in individuals following Western dietary patterns high in fat and low in fiber (NIH).
Populations with diets rich in processed foods and low in plant-based fibers often exhibit higher levels of Bacteroides vulgatus in their gut microbiota. This imbalance can disrupt the production of beneficial short-chain fatty acids and promote pro-inflammatory pathways, increasing the risk of metabolic syndrome and its complications (Cell Host & Microbe).
To support a balanced microbiome and reduce the risk of metabolic syndrome, it is recommended to adopt a diet abundant in whole grains, legumes, fruits, and vegetables while minimizing processed foods and saturated fats. Such dietary patterns foster microbial diversity, enhance the growth of anti-inflammatory bacteria, and help keep levels of Bacteroides vulgatus in check for better metabolic health.
38. Ruminococcus gnavus
Ruminococcus gnavus is a gut bacterium that has been found in increased abundance among individuals with allergic conditions, particularly eczema (atopic dermatitis). Several studies reveal that children and adults with eczema tend to have higher levels of Ruminococcus gnavus in their gut microbiome compared to those without the condition (NIH). This overgrowth is thought to influence immune responses, potentially triggering or intensifying allergic inflammation both in the gut and on the skin.
Research further suggests that Ruminococcus gnavus may produce specific polysaccharides and metabolites that stimulate the immune system, increasing the risk of allergic reactions. Animal and human studies indicate a connection between the presence of this bacterium and heightened responses to allergens, underscoring its potential impact on allergy severity (Journal of Allergy and Clinical Immunology).
Practical allergy management includes tracking flare-ups and identifying dietary or environmental triggers. Incorporating more fiber-rich plant foods can help diversify the gut microbiome, potentially reducing Ruminococcus gnavus overgrowth. Consulting an allergist or dermatologist may be beneficial for those with persistent eczema, as personalized interventions can help manage symptoms more effectively.
39. Megasphaera elsdenii
Megasphaera elsdenii is a lesser-known but important gut bacterium recognized for its ability to convert lactate into short-chain fatty acids (SCFAs), such as butyrate and propionate. These SCFAs are critical for colon health, energy metabolism, and reducing gut inflammation (NIH). By recycling excess lactate, Megasphaera elsdenii helps maintain acid-base balance in the gut and supports the growth of other beneficial microbes.
Recent research has found a higher abundance of Megasphaera elsdenii in elite endurance athletes, suggesting a link between this bacterium and improved exercise capacity. The efficient conversion of lactate to SCFAs may help athletes recover more quickly from intense training by providing additional energy substrates and reducing muscle fatigue (Nature Microbiology).
For those seeking to optimize exercise-related gut health, promoting Megasphaera elsdenii may be beneficial. Consuming a diet rich in fermentable fibers (from fruits, vegetables, and whole grains) and maintaining a consistent exercise routine can help foster a microbiome that supports faster recovery, better energy utilization, and overall digestive harmony.
40. Phascolarctobacterium faecium
Phascolarctobacterium faecium is a noteworthy gut bacterium that specializes in producing propionate, a type of short-chain fatty acid (SCFA) with significant health effects. Propionate is known for its role in regulating appetite and promoting feelings of fullness by influencing satiety hormones such as peptide YY and GLP-1 (NIH). As a result, higher levels of Phascolarctobacterium faecium and propionate production are associated with reduced food intake and better weight management.
Studies have linked an abundance of Phascolarctobacterium faecium to improved metabolic profiles and a lower risk of obesity, likely due to its impact on satiety signaling and energy balance (Cell Metabolism). This bacterium thrives on the fermentation of dietary fibers, particularly those found in fruits, vegetables, and whole grains.
To support Phascolarctobacterium faecium and boost propionate production, focus on a diet rich in diverse, plant-based fibers. Including foods like legumes, oats, apples, and berries can help cultivate this beneficial microbe, contributing to a greater sense of fullness, improved appetite regulation, and overall metabolic health.
41. Odoribacter splanchnicus
Odoribacter splanchnicus is a beneficial gut bacterium known for its pivotal role in producing anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs) and specific secondary bile acids. These metabolites help modulate immune responses, reduce inflammation in the gut, and contribute to the maintenance of a healthy intestinal barrier (NIH). An abundance of Odoribacter splanchnicus has been associated with a lower risk of inflammatory bowel diseases and other chronic inflammatory conditions.
Recent research suggests that polyphenol-rich foods can selectively encourage the growth of Odoribacter splanchnicus. Polyphenols, found in foods such as berries, dark chocolate, green tea, and red wine, serve as prebiotics that beneficially alter the gut microbiota composition (Frontiers in Microbiology). These compounds are metabolized by gut bacteria, leading to the generation of bioactive metabolites that further support anti-inflammatory pathways.
To foster Odoribacter splanchnicus and its anti-inflammatory effects, regularly include a wide variety of polyphenol-rich foods and dietary fibers in your meals. This approach not only benefits the gut environment but also supports systemic health by reducing inflammation and enhancing immune resilience.
42. Bifidobacterium breve
Bifidobacterium breve is a prominent probiotic bacterium frequently used in infant formulas due to its well-established safety and benefits for early-life gut health. Naturally present in the intestines of breastfed infants, Bifidobacterium breve helps establish a balanced microbiome, supports the development of the immune system, and aids in the digestion of milk oligosaccharides (NIH).
Clinical studies have highlighted the protective effects of Bifidobacterium breve against allergic conditions, particularly eczema. Supplementation with this probiotic in infants—either through formula or drops—has been shown to reduce the incidence and severity of atopic dermatitis, likely by strengthening gut barrier function and promoting immune tolerance (Karger). These findings underscore its role in allergy prevention and the importance of early microbial exposures in shaping lifelong health.
For parents, it’s important to choose infant formulas or probiotics that specifically list Bifidobacterium breve with clear strain identification and viable colony-forming units (CFUs). Consulting with a pediatrician before starting any probiotic supplementation ensures safety and appropriateness for the child’s unique health needs, especially in infants with a family history of allergies or immune concerns.
43. Enterobacter cloacae
Enterobacter cloacae is a gut bacterium that, while typically harmless in healthy individuals, can become a significant pathogen in hospital settings. Enterobacter cloacae is known for causing opportunistic infections, particularly in immunocompromised patients, infants, and those with invasive devices such as catheters or ventilators (NIH). Common infections include urinary tract infections, respiratory tract infections, and bloodstream infections, which can be severe and challenging to treat.
One of the most concerning features of Enterobacter cloacae is its capacity to develop resistance to multiple antibiotics, including carbapenems and cephalosporins. This resistance often results from the acquisition of specific genes that encode enzymes capable of breaking down antibiotics, making infections harder to manage and increasing the risk of outbreaks in healthcare settings (CDC).
Strict hospital hygiene practices are essential to prevent the spread of Enterobacter cloacae and other multidrug-resistant organisms. These include rigorous hand hygiene, sterilization of medical equipment, isolation protocols for infected patients, and prudent antibiotic stewardship to minimize the development and transmission of resistance.
44. Peptostreptococcus anaerobius
Peptostreptococcus anaerobius is an anaerobic bacterium commonly found in the human mouth, gut, and skin. While it is a normal resident of these sites, Peptostreptococcus anaerobius can become opportunistic under certain conditions, contributing to skin and dental infections. It is frequently isolated from abscesses, periodontal (gum) disease, and even post-surgical wound infections (NIH).
Warning signs of infection involving Peptostreptococcus anaerobius include persistent swelling, redness, pus formation, foul odor, or pain in the affected area—whether on the skin or in the mouth. In dental settings, it is associated with gingivitis, periodontitis, and dental abscesses, which, if left untreated, can lead to more serious complications such as bone loss or systemic infection (Journal of Clinical Microbiology).
Good oral hygiene is crucial in preventing overgrowth and infection by Peptostreptococcus anaerobius. This includes regular brushing and flossing, routine dental checkups, and prompt treatment of dental issues. For skin health, keeping wounds clean and watching for signs of infection are key. If symptoms persist or worsen, seeking medical or dental care is advised.
45. Clostridium difficile
Clostridium difficile (often referred to as C. diff) is a bacterium that can cause serious health problems when it overgrows in the gut, particularly following antibiotic use. Antibiotics, while targeting harmful bacteria, can disrupt the balance of the gut microbiome and reduce populations of protective microbes, allowing Clostridium difficile to proliferate unchecked (CDC).
Overgrowth of C. difficile leads to the production of toxins that inflame the colon and result in severe colitis, characterized by symptoms such as watery diarrhea, abdominal pain, fever, and sometimes life-threatening complications like toxic megacolon or sepsis (NIH). Elderly individuals, hospitalized patients, and those with weakened immune systems are at particular risk.
Urgent medical care is required if you experience persistent, severe diarrhea (especially after recent antibiotic use), blood or pus in stools, high fever, or signs of dehydration. Early diagnosis and treatment are crucial for preventing severe complications. Treatment may include specific antibiotics targeting C. difficile, probiotics, or, in severe cases, fecal microbiota transplantation to restore healthy gut flora.
46. Bacteroides ovatus
Bacteroides ovatus is a prominent gut bacterium known for its impressive ability to ferment complex plant fibers. Through this fermentation process, Bacteroides ovatus produces beneficial short-chain fatty acids (SCFAs), such as acetate and propionate, which support gut barrier integrity, reduce inflammation, and serve as energy sources for colon cells (NIH).
Vegetarian and plant-based diets are often associated with higher levels of Bacteroides ovatus in the gut, as these eating patterns provide abundant fermentable fibers from whole grains, legumes, fruits, and vegetables. Studies have shown that individuals following vegetarian diets tend to have a more diverse and robust population of fiber-fermenting bacteria, contributing to lower rates of inflammatory and metabolic diseases (Frontiers in Microbiology).
To boost fiber intake and cultivate Bacteroides ovatus, aim for a daily mix of soluble and insoluble fibers. Incorporate foods like oats, beans, lentils, apples, berries, carrots, and leafy greens. Gradually increasing fiber while staying hydrated can help prevent digestive discomfort and maximize the benefits for your gut microbiome.
47. Lactobacillus casei
Lactobacillus casei is a widely researched probiotic known for its supportive role in maintaining and strengthening the gut barrier. By promoting the production of mucus and tight junction proteins, Lactobacillus casei helps preserve intestinal integrity and prevents the entry of harmful pathogens and toxins into the bloodstream (NIH). This gut barrier support is essential for digestive health and overall immune function.
Numerous clinical studies have demonstrated the effectiveness of Lactobacillus casei in preventing and reducing the duration of various types of diarrhea, including antibiotic-associated and infectious diarrhea. In both children and adults, supplementation with L. casei has been linked to faster recovery and fewer gastrointestinal symptoms during and after illness (Cochrane Library).
Yogurt is one of the most accessible dietary sources of Lactobacillus casei. When choosing yogurt, look for products that specifically list L. casei or “live and active cultures” on the label. For those avoiding dairy, some plant-based yogurts are now fortified with this beneficial strain, offering gut health support for a variety of dietary preferences.
48. Enterobacteriaceae family

Enterobacteriaceae is a large and diverse family of bacteria that includes both harmless gut residents and well-known pathogens such as Escherichia coli, Klebsiella, and Salmonella. Members of this family are found naturally in the intestines of humans and animals, where they play roles in digestion and nutrient absorption. However, certain strains can cause infections if they become overrepresented or acquire virulent traits (NIH).
Western diets—characterized by high intake of animal fat, processed foods, and low fiber—are associated with an increased abundance of potentially harmful Enterobacteriaceae in the gut. This imbalance, known as dysbiosis, can contribute to inflammation, metabolic disorders, and susceptibility to infections (Cell Host & Microbe). Overgrowth of this bacterial family has also been linked to a higher risk of gastrointestinal diseases.
Dietary variety, particularly the inclusion of plant-based fibers and diverse whole foods, is crucial for maintaining a balanced gut ecosystem. A diet rich in fruits, vegetables, legumes, and whole grains supports the growth of beneficial bacteria and helps keep Enterobacteriaceae in check, protecting gut and overall health.
49. Turicibacter sanguinis
Turicibacter sanguinis is a lesser-known gut bacterium that has recently emerged as a key player in the regulation of serotonin, a neurotransmitter crucial for mood stability, digestive function, and overall well-being. Serotonin is primarily produced in the gut, and Turicibacter sanguinis appears to influence its synthesis and availability through interactions with host cells and other microbes (Cell).
Animal model studies have demonstrated that the presence of Turicibacter sanguinis can modulate serotonin levels in the gut, impacting both gastrointestinal motility and behavioral outcomes. Mice with higher levels of this bacterium showed increased serotonin production and exhibited reduced anxiety-like behaviors, highlighting the direct link between microbial composition and mood regulation (Nature Molecular Psychiatry).
These findings add to the growing body of evidence supporting the gut-mood connection, also known as the gut-brain axis. Maintaining a healthy and diverse microbiome through a balanced, fiber-rich diet and stress reduction strategies may nurture beneficial microbes like Turicibacter sanguinis, ultimately supporting both digestive and emotional health.
50. Clostridium symbiosum
Clostridium symbiosum is an anaerobic bacterium gaining recognition for its involvement in lipid metabolism within the gut. Emerging studies indicate that Clostridium symbiosum plays a role in breaking down and processing dietary fats, influencing the absorption and storage of lipids in the body (NIH). Alterations in its abundance have been observed in individuals with obesity, suggesting that imbalances in this microbe may contribute to disrupted fat metabolism and weight gain.
Recent research has found that people with higher levels of Clostridium symbiosum in their gut tend to have more favorable lipid profiles and are less likely to develop metabolic syndrome, a condition characterized by increased body fat, high cholesterol, and insulin resistance (Metabolic Syndrome and Related Disorders). While the precise mechanisms are still under investigation, it is clear that this bacterium plays a part in the complex relationship between the microbiome and metabolic health.
To support healthy lipid metabolism and beneficial microbes like Clostridium symbiosum, focus on consuming unsaturated fats from sources such as olive oil, avocados, nuts, seeds, and fatty fish. Reducing processed and saturated fats further encourages a balanced gut microbiome and supports optimal metabolic function.
Conclusion

Understanding the profound impact of gut bacteria on weight, mood, and skin is increasingly urgent as research uncovers their central roles in health and disease. Regular gut health checkups, such as microbiome testing (NIH), and consultations with a registered dietitian (Academy of Nutrition and Dietetics) can help personalize diet and lifestyle strategies for optimal well-being. Remember, gut health is highly individual and should be monitored over time. Medical disclaimer: This article is for informational purposes only and does not substitute for professional medical advice, diagnosis, or treatment. Always consult your healthcare provider before making changes to your health regimen.
Disclaimer
The information provided in this article is for general informational purposes only. While we strive to keep the information up-to-date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability, or availability with respect to the article or the information, products, services, or related graphics contained in the article for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
In no event will we be liable for any loss or damage including without limitation, indirect or consequential loss or damage, or any loss or damage whatsoever arising from loss of data or profits arising out of, or in connection with, the use of this article.
Through this article you are able to link to other websites which are not under our control. We have no control over the nature, content, and availability of those sites. The inclusion of any links does not necessarily imply a recommendation or endorse the views expressed within them.
Every effort is made to keep the article up and running smoothly. However, we take no responsibility for, and will not be liable for, the article being temporarily unavailable due to technical issues beyond our control.