The Hidden Bacteria Link Between Gut Health and Arthritis Flare-Ups
Arthritis now affects over 350 million people worldwide, with recent CDC data indicating a continued rise in cases during 2023-2024. At the same time, studies show that gut microbiome imbalances are present in up to 70% of individuals with autoimmune disorders. The gut’s complex bacterial ecosystem and its interaction with the immune system play a pivotal, yet often overlooked, role in joint inflammation. However, early recognition of the bacterial connection between gut health and arthritis flare-ups remains a significant clinical challenge.
1. Gut Microbiome Basics
The gut microbiome refers to the diverse community of trillions of microorganisms—mainly bacteria, but also viruses, fungi, and protozoa—that inhabit the human digestive tract. These microbes collectively weigh up to 2 kilograms and comprise over 1,000 different bacterial species. Their composition varies between individuals and can be influenced by factors like diet, medication, genetics, and environment. The microbiome is not just a passive inhabitant; it plays a crucial role in digestion, vitamin synthesis, and the maintenance of the gut barrier.
Most importantly, the gut microbiome exerts a profound influence on the immune system. Around 70% of the body’s immune cells reside in the gut, constantly interacting with microbial residents. Beneficial bacteria help regulate immune responses, suppressing chronic inflammation and preventing the immune system from attacking the body’s own tissues. Conversely, an imbalance in the microbiome—known as dysbiosis—can trigger immune dysfunction and heightened inflammation. This link between microbial composition and immune activity is increasingly recognized as a key factor in the development and exacerbation of inflammatory diseases, including arthritis (Nature Reviews Microbiology, 2022).
2. Understanding Arthritis Flare-Ups
Arthritis flare-ups are episodes of intensified joint pain, swelling, stiffness, and reduced mobility that can occur suddenly or develop gradually. These flare-ups can be unpredictable and vary in severity and duration. Flare-ups are particularly common in inflammatory forms of arthritis, such as rheumatoid arthritis (RA), psoriatic arthritis, and gout, but can also affect those with osteoarthritis, albeit less frequently and with different underlying mechanisms.
The immune system is central to these flare-ups. In autoimmune types like RA, the immune system mistakenly targets the synovium—the lining of the joints—causing inflammation and tissue damage. During a flare, immune cells such as T cells and macrophages flood the affected joints, releasing pro-inflammatory cytokines like TNF-α and IL-6. This immune response leads to the hallmark redness, warmth, and swelling of a flare-up. While the triggers are not always clear, stress, infections, and even changes in the gut microbiome have been implicated in provoking these inflammatory responses and worsening arthritis symptoms.
3. The Immune System-Gut Connection
The gut is often described as the body’s largest immune organ, home to a vast array of immune cells continually interacting with the resident microbiota. Specialized immune cells in the gut lining, such as T cells and dendritic cells, sample antigens from gut bacteria to determine whether to mount a defensive response or promote tolerance. This dynamic crosstalk is essential for maintaining immune balance and preventing unnecessary inflammation. When the microbiome is healthy and diverse, it encourages the production of anti-inflammatory compounds and regulatory immune cells (Frontiers in Immunology, 2018).
However, dysbiosis—a disruption in the normal balance of gut bacteria—can skew this interaction. Harmful bacteria may proliferate, while beneficial microbes decline, weakening the intestinal barrier and allowing bacterial fragments and toxins to leak into the bloodstream. This leakage, known as increased intestinal permeability or “leaky gut,” can activate immune cells and trigger systemic inflammation. In genetically susceptible individuals, such as those prone to autoimmune diseases, this immune activation can extend beyond the gut, increasing the risk of inflammatory flare-ups in distant sites like the joints (Nature Reviews Immunology, 2020).
4. Key Bacteria Linked to Arthritis
Recent research has spotlighted certain bacterial species in the gut that are closely linked to a heightened risk of developing arthritis, particularly rheumatoid arthritis (RA). One of the most studied is Prevotella copri, a bacterium found in higher abundance in the intestines of people with new-onset RA compared to healthy individuals. Studies suggest that P. copri may drive inflammation by stimulating immune responses that contribute to joint damage.
Other bacterial groups, such as Collinsella and Eggerthella, have also been associated with increased arthritis activity and gut inflammation (Nature Communications, 2015). Conversely, beneficial bacteria like Bifidobacterium and Lactobacillus are often reduced in people with arthritis, which may deprive the immune system of their anti-inflammatory effects. These shifts in gut microbial composition, known as microbial dysbiosis, can lead to an altered immune environment that favors chronic inflammation. Understanding the specific bacterial culprits involved not only helps identify those at risk but also opens the door for targeted therapies that restore microbial balance and potentially alleviate arthritis symptoms.
5. Leaky Gut Syndrome Explained
Leaky gut syndrome, also known as increased intestinal permeability, refers to a condition where the lining of the small intestine becomes damaged or compromised. Normally, this lining acts as a tight barrier that selectively allows nutrients and water to enter the bloodstream while blocking harmful substances and pathogens. However, when the gut barrier is weakened—often due to inflammation, infections, poor diet, or dysbiosis—tiny gaps develop between the cells lining the gut wall (World Journal of Gastroenterology, 2014).
These gaps permit the passage of larger, potentially toxic molecules, such as undigested food particles, bacterial fragments (like lipopolysaccharides), and inflammatory compounds, into the bloodstream. Once these substances escape the gut, they can provoke an immune response throughout the body. In genetically predisposed individuals, this immune activation may become chronic, leading to systemic inflammation that affects distant organs—including the joints. This process is believed to contribute to the onset and exacerbation of autoimmune conditions like arthritis. Efforts to restore gut barrier integrity, through diet and lifestyle changes, are being explored as strategies to reduce inflammation and support joint health (Frontiers in Immunology, 2021).
6. Microbial Diversity and Inflammation
A diverse gut microbiome is vital for maintaining a healthy immune system and minimizing chronic inflammation. Diversity refers to the richness and even distribution of different bacterial species within the gut. High microbial diversity is often associated with stability and resilience, enabling the microbiome to better resist disruptions that can lead to disease. When the gut harbors a wide variety of beneficial microbes, these bacteria work collectively to outcompete harmful species, produce anti-inflammatory compounds, and strengthen the gut barrier (Nature, 2017).
Conversely, low microbial diversity—often seen in people with modern, highly processed diets or those who have taken frequent antibiotics—has been linked to increased susceptibility to chronic inflammation and autoimmune diseases, including arthritis. Reduced diversity can result in the loss of bacteria that produce short-chain fatty acids (SCFAs), such as butyrate, which play a crucial role in suppressing inflammatory pathways and maintaining gut lining integrity (Frontiers in Immunology, 2018). Promoting a varied gut microbiome through diet and lifestyle may therefore help mitigate systemic inflammation, lower the risk of arthritis flare-ups, and support overall joint health.
7. Dysbiosis: When Balance Tips
Dysbiosis is the scientific term for an imbalance in the gut microbiome, where the proportions of beneficial and harmful bacteria are disrupted. This imbalance can manifest as a loss of microbial diversity, a reduction in health-promoting species, or an overgrowth of pathogenic organisms. Dysbiosis is increasingly recognized as a key driver of many chronic health conditions, especially autoimmune diseases like arthritis (Cells, 2020).
Several factors can contribute to dysbiosis, including long-term antibiotic use, diets low in fiber and high in processed foods, chronic stress, infections, and environmental toxins. When dysbiosis occurs, the gut’s protective functions are compromised. Harmful bacteria may produce substances that damage the intestinal lining or provoke the immune system. At the same time, the decline of beneficial microbes can reduce the production of anti-inflammatory compounds. This environment fosters inflammation and increases the risk of the immune system attacking the body’s own tissues. In arthritis, dysbiosis has been linked to more frequent and severe flare-ups, as well as the progression of joint damage. Understanding and correcting dysbiosis is therefore a promising avenue for managing autoimmune and inflammatory diseases (Nature Communications, 2023).
8. Short-Chain Fatty Acids (SCFAs) Role
Short-chain fatty acids (SCFAs)—primarily acetate, propionate, and butyrate—are beneficial compounds produced when gut bacteria ferment dietary fiber. These SCFAs play a pivotal role in maintaining gut health, regulating immune responses, and reducing inflammation throughout the body. Among them, butyrate is particularly important for nourishing the cells that line the colon, strengthening the intestinal barrier, and preventing the leakage of harmful substances into the bloodstream (Frontiers in Immunology, 2018).
SCFAs exert significant anti-inflammatory effects by promoting the development and function of regulatory T cells, which help keep the immune system in balance and prevent it from attacking the body’s own tissues. They also inhibit the production of pro-inflammatory cytokines that contribute to joint pain and swelling. In the context of arthritis, a robust SCFA-producing microbiome has been linked to milder symptoms and fewer flare-ups. Conversely, individuals with dysbiosis often have reduced SCFA levels, increasing their vulnerability to inflammation and joint damage (Nature Reviews Microbiology, 2022). Supporting SCFA production through a fiber-rich diet is therefore a promising strategy for protecting joint health and managing arthritis symptoms.
9. Antibiotic Use and Flare-Ups
Antibiotics are powerful tools against bacterial infections, but their impact extends beyond eradicating harmful pathogens. These medications often disrupt the delicate balance of the gut microbiome by killing both harmful and beneficial bacteria indiscriminately. This disruption can lead to dysbiosis, which is associated with heightened inflammation and an increased risk of autoimmune flare-ups, including those linked to arthritis (BMJ, 2019).
Research has shown that recent or frequent antibiotic use may precede the onset or exacerbation of autoimmune diseases such as rheumatoid arthritis. By reducing populations of beneficial bacteria—particularly those that produce anti-inflammatory short-chain fatty acids—antibiotics can weaken the gut barrier and allow inflammatory substances to enter the bloodstream. This can trigger immune responses in genetically susceptible individuals, leading to joint pain and swelling. A Nature Reviews Rheumatology study found that people with a history of antibiotic use had a higher risk of developing RA. While antibiotics are sometimes necessary, their use should be carefully evaluated, and strategies to restore gut balance—such as probiotics or dietary changes—should be considered to help prevent arthritis flare-ups.
10. Probiotics and Symptom Relief

Probiotics—live microorganisms that confer health benefits when consumed in adequate amounts—have gained significant attention for their potential to modulate the gut microbiome and support immune health. Several clinical studies suggest that probiotic supplementation may help restore microbial balance, reduce inflammation, and alleviate symptoms in people with arthritis. Specific strains of Lactobacillus and Bifidobacterium have been shown to enhance gut barrier integrity, increase the production of anti-inflammatory short-chain fatty acids, and suppress the activity of pro-inflammatory immune cells (Nutrients, 2020).
For individuals with rheumatoid arthritis or other inflammatory arthritic conditions, regular consumption of probiotics has been linked to reduced joint pain, swelling, and improved physical function. A 2018 randomized controlled trial found that participants taking a daily probiotic supplement experienced significant decreases in disease activity and inflammatory markers compared to those who took a placebo. While not a cure, probiotics represent a promising adjunct therapy that may help manage arthritis symptoms by targeting the gut-immune axis. As always, individuals should consult healthcare professionals before starting supplementation to ensure safety and optimal strain selection.
11. Prebiotics: Feeding the Good Bacteria

Prebiotics are non-digestible fibers and compounds found in certain foods that selectively nourish and stimulate the growth of beneficial gut bacteria. Unlike probiotics, which introduce live organisms into the gut, prebiotics serve as “food” for the existing microbiota, particularly strains such as Bifidobacterium and Lactobacillus. Common prebiotic sources include inulin, fructooligosaccharides (FOS), and resistant starches, which are naturally abundant in foods like chicory root, garlic, onions, leeks, bananas, and whole grains (Nutrients, 2013).
By enhancing the growth of these beneficial microbes, prebiotics boost the production of short-chain fatty acids (SCFAs) such as butyrate, which have well-documented anti-inflammatory effects. Increased SCFA production strengthens the gut lining, reduces intestinal permeability, and helps suppress the immune system’s tendency toward overreaction and chronic inflammation. Some studies indicate that prebiotic supplementation can decrease systemic inflammation and improve symptoms in individuals with autoimmune conditions, including arthritis (Nutrients, 2020). Incorporating prebiotic-rich foods into the diet is a simple and sustainable way to support gut health, foster a balanced microbiome, and potentially reduce the frequency and severity of joint flare-ups.
12. Diet Patterns That Influence Gut Health

The composition of the gut microbiome is profoundly influenced by daily dietary choices. Diets rich in fiber—such as those featuring whole grains, legumes, fruits, and vegetables—promote the growth of beneficial bacteria and boost the production of anti-inflammatory short-chain fatty acids (SCFAs). These nutrients help support a healthy gut barrier, reduce inflammation, and have been linked to improved outcomes in people with arthritis (Nutrients, 2020).
In contrast, processed diets high in refined sugars, unhealthy fats, and low in fiber can disrupt microbial diversity and encourage the growth of pro-inflammatory bacteria. Such diets have been associated with increased intestinal permeability (“leaky gut”), reduced SCFA production, and heightened systemic inflammation—factors that may contribute to the onset or worsening of arthritis symptoms (Cell Research, 2021). Studies have shown that individuals who consume a typical Western diet are more likely to experience gut dysbiosis and more severe arthritis flare-ups compared to those following a Mediterranean or plant-based diet. Prioritizing fiber-rich, minimally processed foods is therefore a foundational strategy for nurturing gut health and supporting joint wellness.
13. Stress, Gut, and Joint Flares
Psychological stress is a well-known trigger for both gut disturbances and arthritis flare-ups. Chronic or acute stress activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to the release of stress hormones such as cortisol. These hormones can negatively impact the composition and function of the gut microbiome by suppressing beneficial bacteria and promoting the growth of harmful species (Frontiers in Immunology, 2017).
Stress-induced changes in the gut microbiota can weaken the intestinal barrier, increasing intestinal permeability and facilitating the movement of inflammatory substances into the bloodstream. This process may spark systemic inflammation and immune dysregulation, which are key contributors to arthritis flare-ups. In fact, clinical studies have found that individuals with rheumatoid arthritis often report more joint pain and swelling during periods of heightened psychological stress (Lupus, 2022). Furthermore, stress may reduce the effectiveness of anti-inflammatory gut bacteria, further exacerbating joint inflammation. Addressing stress through mindfulness, therapy, and lifestyle changes is therefore not only beneficial for mental health but may also help stabilize the gut microbiome and reduce the frequency and intensity of arthritis symptoms.
14. The Role of Fermented Foods

Fermented foods such as yogurt, kefir, sauerkraut, kimchi, miso, and kombucha have been consumed for centuries, not only for their flavors but also for their potential health benefits. Recent scientific studies have confirmed that these foods are rich in live microorganisms—primarily lactic acid bacteria and yeasts—that can enhance the diversity and function of the gut microbiome. Regular consumption of fermented foods has been shown to increase beneficial bacteria, such as Lactobacillus and Bifidobacterium, and to reduce populations of potentially harmful microbes (Cell, 2021).
These improvements in microbial balance are associated with a reduction in chronic inflammation, as observed in both healthy individuals and those with autoimmune or inflammatory conditions. Fermented foods help promote the production of short-chain fatty acids (SCFAs), support gut barrier integrity, and modulate immune responses. One study demonstrated that a diet rich in fermented foods led to lower levels of inflammatory markers and improved overall immune function (Nature Reviews Microbiology, 2022). Incorporating a variety of fermented foods into the diet may thus be a simple, natural way to improve gut health and potentially reduce arthritis flare-ups.
15. Medication Side Effects on Gut Bacteria
Many individuals with arthritis rely on medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs) for symptom control. While these drugs are effective for reducing pain and inflammation, they can also have unintended consequences for gut health. NSAIDs, in particular, are known to damage the protective lining of the gastrointestinal tract, increasing the risk of ulcers, bleeding, and intestinal permeability (World Journal of Gastroenterology, 2012).
Beyond direct mucosal injury, these medications can disrupt the delicate balance of the gut microbiome. Studies have shown that NSAIDs and corticosteroids may reduce populations of beneficial bacteria like Bifidobacterium and Lactobacillus, while promoting the growth of potentially harmful species. DMARDs, including methotrexate, can also alter microbial diversity and function (Frontiers in Medicine, 2021). These changes may worsen gut inflammation, impair the gut barrier, and even contribute to an increased risk of arthritis flare-ups. It is important for individuals taking these medications to be aware of potential gut-related side effects and to discuss strategies for protecting gut health with their healthcare providers.
16. Smoking, Alcohol, and Microbiome Damage
Smoking and excessive alcohol consumption are lifestyle factors well-documented to harm gut health and worsen inflammatory conditions such as arthritis. Tobacco smoke contains toxic chemicals that can directly damage the intestinal lining and alter the composition of gut bacteria. Studies have found that smokers exhibit reduced levels of beneficial microbes like Bifidobacterium and Lactobacillus, along with an increase in pro-inflammatory bacteria (Nutrients, 2020).
Similarly, chronic alcohol intake disrupts the balance of the gut microbiome, impairs gut barrier function, and promotes systemic inflammation. Alcohol can increase the growth of pathogenic bacteria, reduce the diversity of beneficial species, and contribute to a “leaky gut,” allowing inflammatory compounds to enter the bloodstream (Nature Reviews Gastroenterology & Hepatology, 2019). For those with arthritis, these disruptions can aggravate joint pain and trigger more frequent or severe flare-ups. Research shows that individuals who smoke or consume alcohol excessively are at greater risk for developing rheumatoid arthritis and experiencing worse disease progression. Reducing or eliminating tobacco and alcohol use is therefore vital for supporting gut health, improving treatment outcomes, and minimizing arthritis-related inflammation.
17. Childhood Gut Health and Adult Risk
Emerging research suggests that the composition of the gut microbiota in early childhood can have long-lasting effects on immune system development and the risk of autoimmune diseases, including arthritis, later in life. During infancy and early childhood, the gut microbiome is highly malleable and influenced by factors such as mode of birth (vaginal delivery vs. C-section), breastfeeding, antibiotic exposure, diet, and environmental exposures (Nature Medicine, 2019).
Several studies have demonstrated that disruptions to the gut microbiota in early life—such as through frequent antibiotic use or a lack of microbial diversity—can impair immune tolerance and increase susceptibility to autoimmune conditions. For example, children with reduced levels of beneficial bacteria like Bifidobacterium and Lactobacillus have been shown to be at higher risk for developing juvenile idiopathic arthritis and other immune-mediated diseases (Nutrients, 2020). These findings underscore the importance of nurturing a healthy gut microbiome during critical developmental windows. Efforts such as promoting breastfeeding, judicious use of antibiotics, and providing a fiber-rich, diverse diet may help reduce the risk of arthritis and other autoimmune disorders in adulthood.
18. Obesity, Gut Bacteria, and Joint Pain
Obesity is a major risk factor for both the development and worsening of arthritis, and mounting evidence points to the gut microbiome as a critical link in this relationship. Excess body fat is associated with significant alterations in gut bacterial composition, including a reduction in microbial diversity and an increase in pro-inflammatory species such as Firmicutes and Proteobacteria (Nature Medicine, 2020). These changes contribute to a chronic low-grade inflammatory state that can exacerbate joint pain and accelerate the progression of arthritis.
Obesity also promotes increased intestinal permeability (“leaky gut”), allowing bacterial toxins and inflammatory molecules to enter the bloodstream and further stimulate immune responses. This systemic inflammation not only affects joint tissues but may also worsen metabolic health, creating a vicious cycle of inflammation and pain. Studies have found that individuals with obesity and arthritis often have lower levels of beneficial, anti-inflammatory bacteria, such as Bifidobacterium and Lactobacillus (International Journal of Molecular Sciences, 2020). Addressing obesity through dietary changes, physical activity, and gut health support may help reduce both systemic inflammation and arthritis-related joint pain, improving quality of life.
19. Exercise Effects on the Microbiome

Regular physical activity is known to offer a multitude of health benefits, and recent research highlights its positive impact on the gut microbiome. Exercise has been shown to increase the abundance and diversity of beneficial bacteria, including those that produce anti-inflammatory short-chain fatty acids (SCFAs) like butyrate. These changes help strengthen the gut barrier, reduce systemic inflammation, and may lower the risk of autoimmune flare-ups, including arthritis (Journal of Applied Physiology, 2019).
Studies indicate that moderate, consistent exercise can significantly increase the presence of Bifidobacterium and Lactobacillus species, while also reducing pro-inflammatory bacteria. For individuals with arthritis, these microbial shifts are associated with fewer flare-ups, decreased joint pain, and improved overall physical function (Frontiers in Microbiology, 2019). Importantly, the anti-inflammatory effects of exercise extend beyond the gut, influencing immune regulation throughout the body. Activities such as walking, swimming, cycling, and yoga can be particularly beneficial for maintaining joint mobility while supporting gut health. Integrating regular movement into daily routines represents a holistic approach to managing arthritis and enhancing well-being through microbiome modulation.
20. Gender Differences in Gut-Arthritis Link
Growing evidence indicates that gender plays a significant role in shaping the gut microbiome and its influence on arthritis risk and symptoms. Men and women possess distinct hormonal environments, which can affect the composition and activity of gut bacteria. For example, estrogen and other female sex hormones have been shown to foster the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus, and may help suppress inflammatory immune responses (Frontiers in Immunology, 2022).
Conversely, men often have higher levels of certain bacterial species that may promote inflammation. These differences partly explain why autoimmune diseases like rheumatoid arthritis are more prevalent in women, but can also manifest differently between genders (Nature Communications, 2023). Additionally, factors such as diet, stress, and medication use may interact with gender-specific microbiome profiles, further influencing arthritis outcomes. Understanding these distinctions is crucial for developing personalized approaches to arthritis prevention and treatment. Ongoing research continues to explore how targeting the gut microbiome in gender-specific ways could optimize immune health and reduce arthritis flare-ups for both men and women.
21. Vitamin D, Gut Bacteria, and Joints

Vitamin D is well-known for its role in bone health, but it also exerts important effects on the immune system and gut microbiome. Adequate vitamin D levels are associated with a more balanced and diverse population of gut bacteria, including increased abundance of beneficial strains such as Bifidobacterium and Lactobacillus. This vitamin helps maintain the integrity of the intestinal barrier, reducing permeability and the likelihood of inflammatory substances entering the bloodstream (Frontiers in Immunology, 2018).
Vitamin D also modulates immune function by promoting the development of regulatory T cells and suppressing the production of pro-inflammatory cytokines. These effects are particularly relevant to autoimmune conditions like arthritis, where chronic inflammation damages joint tissues. Studies have shown that individuals with low vitamin D status are more likely to experience increased arthritis severity and more frequent flare-ups (Nutrients, 2020). Supplementing with vitamin D—or ensuring sufficient sun exposure and dietary intake—may help optimize gut microbiome composition, support immune resilience, and reduce inflammation. Consulting a healthcare provider can determine if supplementation is necessary for individual joint and gut health needs.
22. Antibiotic Resistance and Gut Health
The widespread use and sometimes misuse of antibiotics has led to a global increase in antibiotic-resistant bacteria, posing significant challenges for healthcare management—including in individuals with arthritis. When antibiotics are used excessively or inappropriately, they not only disrupt the balance of beneficial gut microbes but also foster the emergence of resistant strains that can persist in the microbiome (CDC, 2024).
Antibiotic-resistant bacteria can outcompete beneficial species, leading to prolonged dysbiosis, impaired gut barrier function, and chronic inflammation. For people with arthritis, this can mean a heightened risk of infection, more severe disease flares, and reduced effectiveness of standard treatments if infections occur. Moreover, the presence of resistant bacteria can complicate the use of immunosuppressive therapies often needed in autoimmune arthritis, as these treatments can further increase susceptibility to difficult-to-treat infections (Frontiers in Microbiology, 2017). Limiting unnecessary antibiotic use, choosing targeted therapies when required, and supporting gut health through probiotics and diet are critical steps to both prevent resistance and manage arthritis more effectively. Public health initiatives continue to emphasize antibiotic stewardship to protect the gut microbiome and overall patient health.
23. Fecal Microbiota Transplantation (FMT)
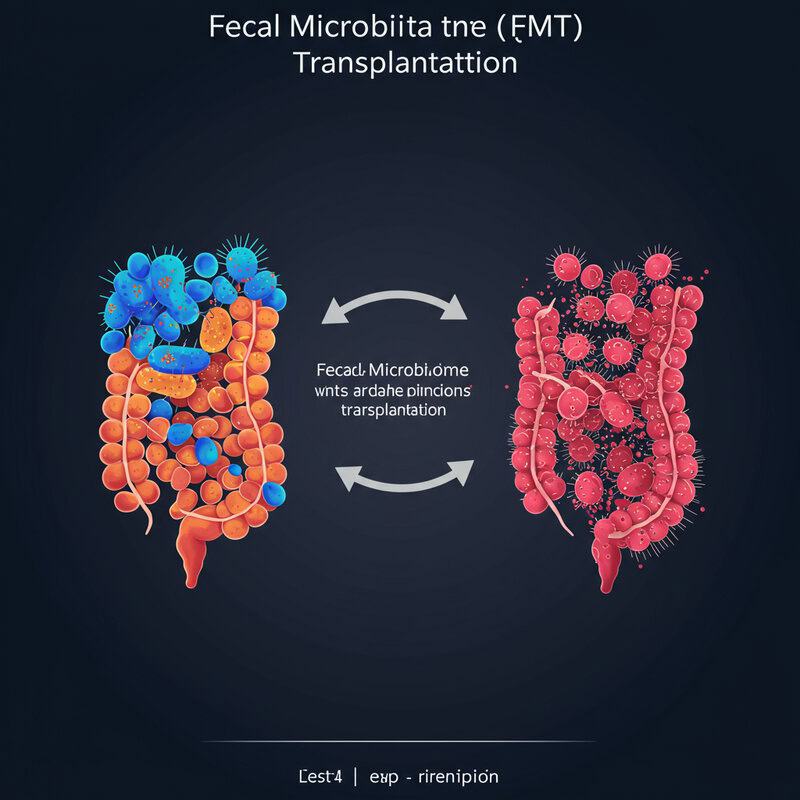
Fecal microbiota transplantation (FMT) is an innovative medical procedure that involves transferring stool from a healthy donor into the gastrointestinal tract of a patient. The goal is to introduce a robust and diverse community of beneficial microbes to restore gut balance, especially in cases of severe dysbiosis. While FMT has become a widely accepted treatment for recurrent Clostridioides difficile infections, research is now exploring its potential in managing autoimmune and inflammatory conditions, including forms of arthritis (Frontiers in Immunology, 2022).
Preliminary studies suggest that FMT can improve gut microbial diversity, enhance the production of anti-inflammatory short-chain fatty acids, and reduce systemic inflammation—all of which are relevant to autoimmune disease management. In rheumatoid arthritis and other related conditions, FMT has shown promise in modulating immune responses and alleviating symptoms, though more large-scale clinical trials are needed to confirm safety and efficacy (British Society for Rheumatology). As FMT technology advances, it may offer a personalized approach to restoring gut health and reducing arthritis flare-ups, particularly for those who have not responded to conventional treatments.
24. Allergies, Asthma, and Gut Microbes
Research has increasingly highlighted the interconnectedness between allergic conditions—such as allergies and asthma—and the gut microbiome. Individuals with allergies or asthma often display altered gut bacterial compositions, marked by reduced diversity and lower levels of beneficial microbes like Bifidobacterium and Lactobacillus. These imbalances may contribute to immune system hyperactivity and increased inflammation, which are also core features of autoimmune conditions like arthritis (Scientific Reports, 2020).
Studies suggest that the same gut-derived immune signals implicated in allergy and asthma development may also influence the risk of autoimmune diseases. For example, dysbiosis can promote the overproduction of immunoglobulin E (IgE) and pro-inflammatory cytokines, which are involved in both allergic reactions and joint inflammation (Frontiers in Microbiology, 2017). This cross-talk between gut microbes and the immune system may partly explain why individuals with allergic diseases are at higher risk for developing arthritis and other immune-mediated conditions. Strategies to restore healthy gut microbiota—such as probiotics, prebiotics, and dietary modifications—could therefore play a role in preventing or managing both allergic and arthritic disorders.
25. Environmental Toxins and Gut Health
Exposure to environmental toxins—including pesticides, heavy metals, air pollutants, and industrial chemicals—can have profound effects on gut health and immune function. These pollutants may enter the body through food, water, or air and disrupt the delicate balance of the gut microbiome. Studies have shown that toxins like glyphosate (a common pesticide) and particulate matter from air pollution can reduce populations of beneficial bacteria while encouraging the growth of pathogenic species.
This microbial imbalance, or dysbiosis, can weaken the intestinal barrier and increase systemic inflammation—key factors in the development and exacerbation of autoimmune and inflammatory conditions such as arthritis. Additionally, some toxins directly trigger immune responses or accumulate in joint tissues, further contributing to pain and swelling. Research indicates that individuals living in areas with high environmental pollution levels often have higher rates of inflammatory diseases, including rheumatoid arthritis (International Journal of Environmental Research and Public Health, 2021). Minimizing exposure to environmental toxins, choosing organic foods when possible, and supporting detoxification through a healthy diet may help protect gut health and reduce arthritis-related inflammation.
26. Sleep Quality and Microbiome Health

Mounting research underscores the importance of sleep quality in maintaining a healthy gut microbiome and managing inflammatory conditions like arthritis. Poor or insufficient sleep can disrupt the balance of gut bacteria, reducing beneficial species such as Bifidobacterium and Lactobacillus while promoting the growth of pro-inflammatory microbes (Nature Communications, 2017). These changes can weaken the gut barrier, increase intestinal permeability, and allow inflammatory molecules to enter the bloodstream, escalating systemic inflammation.
For individuals with arthritis, poor sleep is linked to more frequent and severe flare-ups, heightened pain sensitivity, and diminished response to treatment. Studies show that sleep disturbances can exacerbate joint inflammation through both direct effects on immune regulation and indirect impacts via the gut microbiome (Frontiers in Neuroscience, 2021). Conversely, improving sleep quality—through consistent sleep schedules, stress reduction, and good sleep hygiene—has been shown to enhance microbial diversity and reduce inflammatory markers. Prioritizing restful sleep is thus a crucial strategy for supporting gut health and optimizing arthritis management, providing a holistic approach to reducing flare-ups and improving overall well-being.
27. Artificial Sweeteners and Microbial Balance
Artificial sweeteners such as aspartame, sucralose, and saccharin are widely used as sugar substitutes, but growing evidence suggests they may negatively impact gut microbiota and contribute to inflammation. Studies have shown that these sweeteners can disrupt the composition and function of gut bacteria, reducing beneficial species while promoting the growth of bacteria linked to metabolic and inflammatory disorders (Nature, 2014).
Animal and human research indicates that artificial sweeteners may impair glucose metabolism and increase intestinal permeability, a phenomenon associated with “leaky gut.” This allows bacterial endotoxins to enter the bloodstream, triggering immune responses that can contribute to systemic and joint inflammation—key concerns for individuals with arthritis. One 2022 review in Frontiers in Nutrition highlights the association between artificial sweetener consumption, altered gut bacteria, and heightened inflammatory markers. For those managing arthritis or at risk for autoimmune conditions, limiting artificial sweetener intake may help preserve a balanced gut microbiome, reduce chronic inflammation, and support joint health. Opting for natural sweeteners and focusing on whole, unprocessed foods is a practical approach to maintaining microbial balance and reducing flare-ups.
28. Autoimmune Triggers in the Gut
The gut is a key battleground for immune system regulation, and certain gut-derived molecules have been identified as potential triggers for autoimmune diseases, including various forms of arthritis. Among the most significant are bacterial components such as lipopolysaccharides (LPS), which are found in the outer membrane of gram-negative bacteria. When intestinal permeability increases—a condition often called “leaky gut”—these molecules can cross into the bloodstream and provoke strong immune responses (Frontiers in Immunology, 2021).
LPS and other bacterial byproducts can activate immune cells and stimulate the release of pro-inflammatory cytokines, which not only escalate systemic inflammation but may also direct immune attacks against joint tissues. In genetically susceptible individuals, this process may trigger or worsen autoimmune arthritis, such as rheumatoid arthritis or psoriatic arthritis. Other gut-derived molecules, such as certain peptides and metabolites resulting from microbial fermentation, have also been implicated in modulating immune responses and influencing disease risk (Nature Reviews Microbiology, 2022). Understanding these triggers highlights the importance of maintaining gut barrier integrity and microbial balance as strategies for preventing or managing autoimmune flare-ups.
29. Personalized Nutrition for Gut Health

The concept of personalized nutrition is gaining momentum as researchers recognize that each individual’s gut microbiome is unique and can influence their susceptibility to inflammation and arthritis flares. Advances in microbiome sequencing and nutrigenomics now allow for tailored dietary recommendations based on a person’s specific gut bacterial composition, genetics, and health goals (Nature Reviews Gastroenterology & Hepatology, 2021).
Personalized nutrition plans often focus on optimizing the intake of fiber, prebiotics, probiotics, and anti-inflammatory foods that specifically support beneficial microbes and address individual imbalances. For some, increasing resistant starches or particular plant-based foods may foster the growth of SCFA-producing bacteria, while others may benefit from targeted probiotic supplementation or elimination of dietary triggers. Studies show that such individualized approaches can improve gut microbial diversity, reduce systemic inflammation, and decrease the frequency and severity of arthritis flare-ups (Frontiers in Nutrition, 2022). As gut microbiome testing becomes more accessible, personalized nutrition is poised to become a cornerstone of arthritis management, empowering individuals to make evidence-based dietary choices that enhance both gut and joint health.
30. Ethnicity, Geography, and Microbiome Differences
Gut microbiome composition is profoundly influenced by ethnicity, geography, culture, and genetic background, which in turn can impact susceptibility to autoimmune conditions like arthritis. Dietary habits, traditional foods, environmental exposures, and even local pathogens all contribute to distinct microbial profiles among populations globally (Nature, 2016). For example, individuals in rural African or Asian communities, who typically consume more fiber-rich, plant-based diets, often have a greater abundance of beneficial bacteria and higher microbial diversity than those in urban Western settings.
Genetic factors also play a role in shaping an individual’s gut microbiome and immune responses. Some populations may be genetically predisposed to harbor certain bacterial strains that influence inflammation and disease risk. Additionally, migration and acculturation can rapidly alter the gut microbiome, sometimes leading to an increased risk of inflammatory diseases as traditional dietary patterns shift (Cell, 2018). Understanding these cultural, regional, and genetic influences is critical for developing targeted, culturally sensitive interventions to optimize gut health and minimize arthritis risk. This knowledge also supports the move toward more personalized and population-specific approaches in arthritis prevention and management.
31. Gut Viruses and Arthritis
While much attention has focused on bacteria, the human gut is also home to a diverse population of viruses, particularly bacteriophages—viruses that infect and regulate bacterial populations. Recent research suggests that these gut viruses, collectively termed the virome, may significantly influence both gut microbial balance and systemic immune responses (Nature Reviews Microbiology, 2021).
Bacteriophages can modulate the abundance and activity of specific bacterial species, indirectly affecting the production of anti-inflammatory or pro-inflammatory molecules in the gut. Disruptions in the virome have been linked to increased bacterial dysbiosis and heightened intestinal inflammation. Some studies now suggest that altered gut viral populations may play a role in autoimmune diseases, including arthritis, by promoting the proliferation of pathogenic bacteria and the release of inflammatory mediators (Frontiers in Microbiology, 2021). Furthermore, bacteriophages themselves may directly interact with the host immune system, triggering or modulating immune responses relevant to joint health. As the science of the gut virome advances, understanding the interplay between viruses, bacteria, and immunity may open new avenues for diagnosing and managing arthritis and other inflammatory conditions.
32. Fungi in the Gut Microbiome
Beyond bacteria and viruses, the gut microbiome also includes a complex community of fungi, collectively known as the mycobiome. Although fungi represent a smaller fraction of the gut’s microbial population, they play important roles in immune regulation and overall gut health. Common fungal genera found in the gut include Candida, Saccharomyces, and Malassezia (Nature Reviews Microbiology, 2019).
The mycobiome interacts with both the host’s immune system and bacterial residents. Certain fungi can influence immune responses, promoting either inflammation or tolerance depending on their abundance and the host’s genetic background. Dysbiosis of the gut mycobiome—such as overgrowth of Candida species—has been linked to increased intestinal permeability, heightened immune activation, and the development or worsening of inflammatory diseases, including arthritis (Frontiers in Immunology, 2020). Imbalances in the mycobiome may contribute to joint pain and swelling by triggering the release of pro-inflammatory cytokines. Ongoing research is exploring how targeted interventions, such as antifungal therapies or dietary modifications, might help restore fungal balance, support immune health, and reduce the risk of arthritis flare-ups.
33. Gut Bacteria and Pain Perception
Emerging research reveals that gut bacteria not only modulate inflammation but also play a direct role in shaping pain perception, particularly in individuals with arthritis. Certain microbial populations can influence the production of neurotransmitters and bioactive compounds—such as serotonin, gamma-aminobutyric acid (GABA), and short-chain fatty acids (SCFAs)—that communicate with the central nervous system and affect pain signaling (Journal of Neuroinflammation, 2020).
Animal and human studies suggest that dysbiosis, or a disrupted gut microbiome, is associated with heightened pain sensitivity and increased joint discomfort. For example, decreases in beneficial bacteria like Lactobacillus and Bifidobacterium have been linked to lower levels of SCFAs, which possess analgesic and anti-inflammatory properties. Conversely, an overgrowth of pro-inflammatory bacteria may enhance the release of cytokines that sensitize pain receptors in joint tissues (Frontiers in Neuroscience, 2021). These findings highlight the gut-joint-brain axis as an important target for arthritis management. Modulating the microbiome through diet, probiotics, or prebiotics could offer a novel approach to alleviating pain and improving quality of life for those living with arthritis.
34. The Role of Fiber Supplements
Fiber supplements—such as psyllium husk, inulin, and resistant starch—are increasingly recognized for their potential to support gut health and reduce inflammation, including that associated with arthritis. Dietary fiber serves as a primary fuel source for beneficial gut bacteria, enabling them to ferment these fibers and produce short-chain fatty acids (SCFAs) like butyrate, acetate, and propionate (Nutrients, 2020). These SCFAs help to regulate immune responses, maintain the integrity of the gut barrier, and suppress the production of pro-inflammatory cytokines.
Clinical studies have demonstrated that supplementing with various forms of dietary fiber can increase microbial diversity and SCFA production, both of which are linked to lower systemic inflammation and improved arthritis symptoms. For individuals struggling to meet fiber needs through food alone, supplements can provide a convenient and effective way to nurture a healthy gut microbiome. A 2021 review in Frontiers in Immunology highlighted that fiber supplementation may reduce the frequency and severity of arthritis flare-ups and enhance overall quality of life. As with any supplement, it is advisable to consult a healthcare provider to determine the most appropriate type and dosage for individual needs.
35. Gut Bacteria and Mental Health in Arthritis
The relationship between gut bacteria and mental health is especially relevant for individuals with arthritis, who are at increased risk for depression and anxiety. The gut-brain axis—a bidirectional communication network linking the gut microbiome, immune system, and central nervous system—plays a crucial role in mood regulation. Disruptions in gut microbial balance, or dysbiosis, can alter the production of neurotransmitters such as serotonin and GABA, which influence emotional well-being (Nature Reviews Gastroenterology & Hepatology, 2019).
Studies have shown that people with arthritis often exhibit both increased intestinal permeability and altered gut bacterial composition, which can elevate systemic inflammation and impact brain function. Chronic inflammation has been linked to higher rates of depression and anxiety in this population. Furthermore, beneficial bacteria like Lactobacillus and Bifidobacterium are associated with reduced stress and improved mood, while pro-inflammatory microbes may exacerbate psychological symptoms (Frontiers in Psychiatry, 2022). Addressing gut health through diet, probiotics, and lifestyle modifications may help alleviate both joint symptoms and mental health challenges, underscoring the importance of a holistic approach to arthritis care.
36. Early Warning Signs in the Gut
Digestive symptoms often serve as early warning signs of underlying gut imbalances that may precede or accompany arthritis flare-ups. Many individuals with autoimmune or inflammatory arthritis report experiencing gastrointestinal (GI) issues before the onset of joint symptoms. Common digestive complaints include bloating, gas, abdominal discomfort, irregular bowel movements (constipation or diarrhea), heartburn, and unexplained changes in stool consistency (Nutrients, 2020).
These symptoms can indicate increased intestinal permeability (“leaky gut”), dysbiosis, or inflammation within the gut lining. In some cases, these GI disturbances are linked to food intolerances or small intestinal bacterial overgrowth (SIBO), both of which can elevate systemic inflammation and contribute to arthritis flare-ups. Studies show that changes in the gut microbiome often occur months or even years before a formal arthritis diagnosis, highlighting the importance of monitoring digestive health as part of early intervention strategies (Frontiers in Immunology, 2021). Recognizing and addressing these gut-related symptoms promptly—with dietary changes, probiotics, or medical evaluation—may help prevent or reduce the severity of future joint inflammation and improve overall quality of life.
37. Gut-Based Biomarkers for Arthritis
Ongoing research is increasingly focused on developing gut-based biomarkers that could revolutionize the prediction and monitoring of arthritis flare-ups. Scientists are investigating stool tests that analyze the composition of gut bacteria, fungal species, and metabolites, aiming to identify microbial patterns or specific biomarkers that correlate with increased risk of joint inflammation (Nature, 2022).
For example, elevated levels of Prevotella copri or a reduction in beneficial Bifidobacterium and Lactobacillus have been linked to early-stage rheumatoid arthritis. Other research highlights the potential of detecting short-chain fatty acid (SCFA) deficiencies or increased inflammatory markers in stool as early warning indicators for flare-ups (Frontiers in Medicine, 2021). By leveraging these microbial signatures, clinicians may eventually be able to anticipate arthritis activity, personalize treatment plans, and intervene before severe symptoms arise. While more validation is needed before these tests become routine in clinical practice, the development of gut-based biomarkers represents a promising frontier in the proactive management of autoimmune and inflammatory arthritis.
38. Gastrointestinal Infections and Flare Risk
Gastrointestinal infections—caused by bacteria, viruses, or parasites—can have a profound, if sometimes temporary, impact on the gut microbiome. Episodes of gastroenteritis often disrupt the delicate balance of gut bacteria, causing a reduction in beneficial species and an overgrowth of opportunistic pathogens (Frontiers in Cellular and Infection Microbiology, 2021). This disturbance, known as dysbiosis, can weaken the intestinal barrier, increase inflammation, and facilitate the passage of microbial fragments into the bloodstream.
For individuals with autoimmune arthritis, such disruptions can trigger or intensify joint flare-ups. The immune system may overreact, targeting not only the infection but also joint tissues in a process sometimes described as “reactive arthritis.” Studies have shown that even after the resolution of acute infection, the microbiome may take weeks or months to fully recover, leaving a window of heightened flare risk (American College of Rheumatology, 2021). Preventative strategies—such as maintaining gut health through diet, hydration, and, when appropriate, probiotics—may reduce the risk of infection-induced flares. Prompt medical attention for gastrointestinal illness is also crucial for those with a history of arthritis or immune dysfunction.
39. The Hygiene Hypothesis and Autoimmunity

The hygiene hypothesis posits that reduced exposure to microbes during early childhood—due to modern sanitation, smaller family sizes, and less contact with animals—may impair the development of a balanced immune system. This lack of microbial diversity in formative years is thought to increase susceptibility to autoimmune diseases, including various forms of arthritis, later in life (Clinical Reviews in Allergy & Immunology, 2014).
Children raised in ultra-clean environments typically have less diverse gut microbiota, which may hinder the education of their immune systems. Without exposure to a broad range of microbes, the immune system may not learn to distinguish harmless antigens from true pathogens, increasing the risk of immune dysregulation and chronic inflammation. Studies have observed that children growing up on farms, or those with frequent outdoor play and animal contact, tend to have lower rates of autoimmune and allergic diseases—including juvenile arthritis—compared to those in urban settings (Nature Reviews Immunology, 2006). These findings emphasize the importance of early-life microbial exposures for immune resilience and suggest that fostering a diverse gut microbiome from childhood could help reduce arthritis risk in adulthood.
40. Gut Bacteria and Medication Absorption
The gut microbiome plays a significant role in the metabolism, absorption, and efficacy of many medications, including those used to treat arthritis. Gut bacteria can chemically modify drugs, influencing how much of the active compound is absorbed, how quickly it is broken down, and the likelihood of experiencing side effects (Nature Reviews Gastroenterology & Hepatology, 2020). For example, certain bacterial species can inactivate or enhance the effects of medications such as methotrexate, sulfasalazine, and biologic agents commonly prescribed for rheumatoid and psoriatic arthritis.
Individual differences in gut microbiome composition may partly explain why some patients respond well to specific arthritis drugs while others do not, or why side effects such as gastrointestinal upset or liver toxicity may occur. Disrupted microbiota, due to antibiotics or chronic inflammation, can alter drug metabolism and absorption, potentially reducing medication effectiveness or increasing toxicity (Frontiers in Medicine, 2021). Understanding the interplay between gut bacteria and medications is guiding the development of personalized treatment approaches, where microbiome profiling could help predict drug responses and minimize adverse effects. Supporting gut health may therefore be a key factor in optimizing arthritis therapy outcomes.
41. Omega-3 Fatty Acids and Microbiome Modulation

Omega-3 fatty acids, found abundantly in fatty fish (such as salmon, mackerel, and sardines), flaxseeds, and walnuts, are renowned for their anti-inflammatory properties. Recent research indicates that omega-3s also play a beneficial role in modulating the gut microbiome, promoting the abundance of health-supporting bacteria like Bifidobacterium and Lactobacillus (Nutrients, 2020). These shifts in microbial composition are associated with increased production of short-chain fatty acids (SCFAs), which help suppress systemic inflammation and maintain gut barrier integrity.
Clinical studies have shown that dietary supplementation with omega-3 fatty acids can reduce the frequency and severity of arthritis flare-ups, likely through both direct immune modulation and indirect effects on gut bacteria (Frontiers in Immunology, 2021). Omega-3s have been found to lower levels of pro-inflammatory cytokines and improve symptoms in patients with rheumatoid arthritis and other inflammatory joint disorders. Including omega-3-rich foods or supplements in the diet may therefore offer a dual benefit: supporting a healthy gut microbiome and reducing arthritis-related inflammation. As always, individuals should consult their healthcare provider for personalized recommendations and dosing guidance.
42. Intermittent Fasting and Gut Health

Intermittent fasting—the practice of cycling between periods of eating and fasting—has gained popularity for its potential health benefits, including weight management, metabolic health, and inflammation reduction. Recent studies reveal that intermittent fasting can also positively influence gut microbiome composition, enhancing the abundance and diversity of beneficial bacteria while reducing those linked to inflammation (Nature Communications, 2019).
Research suggests that fasting periods create a gut environment conducive to the growth of SCFA-producing microbes, such as Faecalibacterium and Akkermansia. These bacteria help strengthen the gut barrier, reduce intestinal permeability, and lower the production of pro-inflammatory cytokines. Several animal and human studies have demonstrated that intermittent fasting can reduce markers of systemic inflammation and improve outcomes in autoimmune and inflammatory diseases, including arthritis (Frontiers in Immunology, 2021). While intermittent fasting is not suitable for everyone, and should be undertaken with medical guidance, it offers a promising lifestyle approach to support microbial balance, decrease flare risk, and improve joint health through gut-mediated mechanisms.
43. Synbiotics: Combining Probiotics and Prebiotics

Synbiotics are supplements or foods that combine both probiotics (live beneficial bacteria) and prebiotics (non-digestible fibers that feed these bacteria), designed to maximize the positive impact on gut health. By providing both the microbes and their preferred fuel source, synbiotics can enhance the survival, colonization, and activity of probiotics in the gastrointestinal tract (Nutrients, 2018).
Research shows that synbiotic supplementation can more effectively restore microbial balance, increase the production of anti-inflammatory short-chain fatty acids (SCFAs), and strengthen the gut barrier compared to probiotics or prebiotics alone. In individuals with autoimmune and inflammatory conditions such as arthritis, clinical studies have demonstrated that synbiotics can reduce systemic inflammation, improve gut barrier integrity, and alleviate joint pain and stiffness (Frontiers in Medicine, 2021). Popular synbiotic combinations include strains of Lactobacillus or Bifidobacterium paired with inulin, fructooligosaccharides (FOS), or galactooligosaccharides (GOS). As the science of the gut microbiome advances, synbiotics represent a promising, holistic approach to supporting gut health and potentially reducing arthritis flare-ups by targeting the microbiome from multiple angles.
44. Role of Hydration in Gut and Joint Health

Proper hydration is fundamental for maintaining both gut and joint health, yet its impact on the gut microbiome is often overlooked. Adequate water intake helps facilitate digestion, supports the movement of food and waste through the intestines, and maintains the optimal environment for beneficial gut bacteria to thrive. Dehydration, on the other hand, can slow gastrointestinal motility, contribute to constipation, and create conditions that favor the growth of harmful bacteria over beneficial ones (Nutrients, 2021).
For those with arthritis, staying well-hydrated is doubly important. Water not only aids in flushing out inflammatory byproducts and supporting joint lubrication, but it also helps maintain the integrity of the gut barrier, reducing the risk of increased intestinal permeability (“leaky gut”) and systemic inflammation. Research indicates that individuals with higher fluid intake tend to have greater microbial diversity and lower levels of inflammatory markers (Frontiers in Nutrition, 2022). Choosing water and limiting dehydrating beverages like sugary drinks and excessive caffeine can further enhance gut and joint function. Ultimately, consistent hydration is an easy yet powerful strategy for supporting microbiome resilience and overall arthritis management.
45. Seasonal Changes and Microbiome Fluctuations

Emerging evidence suggests that seasonal changes can significantly influence the composition and activity of the gut microbiome, which may in turn affect arthritis symptoms and flare risk. Factors such as temperature, humidity, sunlight exposure, and—most notably—seasonal shifts in diet all contribute to microbial fluctuations throughout the year (Cell, 2014).
Studies have found that people in temperate climates often experience greater microbial diversity during the summer and autumn, when fresh fruits, vegetables, and fiber-rich foods are more available. In contrast, winter diets—characterized by higher fat, lower fiber, and more processed foods—may decrease beneficial bacteria and increase the prevalence of pro-inflammatory species. These seasonal microbiome changes can influence immune function, gut barrier integrity, and systemic inflammation. For individuals with arthritis, some report more frequent or severe joint flares during colder months, possibly due to both environmental and microbiome-mediated effects (Frontiers in Microbiology, 2022). Understanding these patterns underscores the importance of maintaining a balanced, fiber-rich diet year-round and adapting lifestyle habits to support gut health, regardless of the season.
46. The Role of Polyphenols

Polyphenols are naturally occurring compounds found in a wide range of plant-based foods, including berries, tea, coffee, cocoa, nuts, olive oil, and colorful fruits and vegetables. These compounds are celebrated for their potent antioxidant and anti-inflammatory properties, but recent research also highlights their impressive ability to modulate the gut microbiome (Nutrients, 2018).
Polyphenols act as prebiotics, selectively nourishing beneficial bacteria such as Bifidobacterium and Lactobacillus while inhibiting the growth of pathogenic microbes. Their fermentation by gut bacteria leads to the production of short-chain fatty acids (SCFAs) and other bioactive metabolites that help regulate immune responses, strengthen the intestinal barrier, and reduce systemic inflammation. Studies have demonstrated that diets rich in polyphenols are linked to increased microbial diversity and lower levels of inflammatory markers, which may help ease arthritis symptoms (Frontiers in Nutrition, 2021). Incorporating polyphenol-rich foods into the daily diet is a delicious and effective way to optimize gut health, support immune balance, and potentially reduce the frequency and severity of arthritis flare-ups.
47. Travel, Diet Change, and Flare-Ups

Travel often brings exciting new experiences, but it can also lead to significant dietary changes that disrupt the gut microbiome and potentially trigger arthritis flare-ups. When individuals travel, they may consume more processed foods, unfamiliar ingredients, or different water sources—each of which can alter the delicate balance of gut bacteria (Nature, 2016). Even short-term dietary shifts have been shown to rapidly change the composition of the gut microbiota, sometimes reducing populations of beneficial microbes while allowing opportunistic or pathogenic bacteria to proliferate.
This microbial imbalance, or dysbiosis, can weaken the gut barrier, increase inflammation, and trigger immune responses that may exacerbate joint pain and swelling. Travel-related stress, sleep disruption, and altered physical activity can further contribute to gut disturbances and immune dysregulation (Frontiers in Microbiology, 2017). For those with arthritis or autoimmune conditions, it’s helpful to plan ahead by incorporating fiber-rich snacks, staying hydrated, and maintaining as much dietary consistency as possible. These strategies can help support microbial resilience, reduce the risk of flare-ups, and ensure that travel remains a positive experience for both gut and joint health.
48. Urban vs. Rural Microbiome Differences

Research reveals substantial differences in the gut microbiome profiles of individuals living in urban versus rural environments, with important implications for inflammatory conditions like arthritis. Rural populations, especially those in non-industrialized regions, typically consume diets richer in fiber, fermented foods, and minimally processed ingredients. This lifestyle supports greater microbial diversity and higher levels of beneficial bacteria such as Bifidobacterium and Prevotella (Nature, 2016).
In contrast, urban dwellers often face reduced microbial exposure due to processed diets, increased antibiotic use, and less contact with soil, animals, and nature. These factors can result in lower diversity and a gut environment that favors pro-inflammatory microbes (Cell, 2018). This urban-rural divide may explain, in part, higher rates of autoimmune and inflammatory diseases—including arthritis—in urbanized societies. The lack of microbial diversity in city living is associated with increased gut permeability and systemic inflammation. Encouraging urban residents to adopt more fiber-rich, whole-food diets, and to engage in nature-based activities, may help bridge the microbiome gap and reduce arthritis risk or severity.
49. Emerging Therapies Targeting the Microbiome

With growing recognition of the gut microbiome’s role in arthritis, researchers are developing emerging therapies designed to modify gut bacteria and manage inflammation more effectively. Beyond traditional probiotics and prebiotics, new strategies include precision probiotics—tailored microbial strains chosen for their anti-inflammatory properties—and next-generation synbiotics that combine specific bacteria with targeted prebiotic fibers for enhanced efficacy (Nature Reviews Rheumatology, 2022).
Other innovative approaches are exploring the use of bacteriophage therapy, which employs viruses that selectively target and eliminate harmful bacteria, thereby restoring microbial balance without disturbing beneficial species. Fecal microbiota transplantation (FMT), already used for recurrent C. difficile infections, is being studied for its potential to treat autoimmune arthritis by re-establishing a healthy microbiota (Frontiers in Immunology, 2022). Additionally, researchers are investigating postbiotics—bioactive compounds produced by beneficial bacteria—that may directly modulate immune responses and reduce joint inflammation. As personalized medicine advances, therapies based on individual microbiome profiles are likely to become central in arthritis management, offering new hope for patients seeking alternatives to conventional treatments.
50. When to Seek Medical Advice
Recognizing when to seek medical advice is crucial for individuals experiencing gut or joint symptoms, especially given the complex relationship between the microbiome and arthritis. Red flags that warrant prompt evaluation include persistent or severe joint pain, swelling, or stiffness—particularly if these symptoms are accompanied by redness, warmth, or reduced range of motion (Arthritis Foundation). Gastrointestinal warning signs such as ongoing abdominal pain, unexplained weight loss, blood in the stool, chronic diarrhea or constipation, and new or worsening digestive complaints should not be ignored.
Additional concerns include fevers, night sweats, unexplained fatigue, or signs of infection, as these may indicate a systemic inflammatory process or complication. For those with a history of autoimmune disease, acute changes in symptoms or the development of new issues should prompt consultation with a healthcare provider (CDC: Arthritis Symptoms). Early medical assessment can facilitate timely diagnosis, guide effective treatment, and help prevent complications. Open communication about both gut and joint health enables clinicians to address the underlying connections and optimize management strategies for long-term well-being.
Conclusion
The evolving science around the gut-arthritis relationship underscores an urgent need for greater awareness and proactive management. Maintaining a healthy, diverse gut microbiome can reduce inflammation and potentially ease arthritis symptoms, making diet, lifestyle, and environmental choices more important than ever. Individuals experiencing persistent digestive or joint issues should consider discussing gut health screenings with their healthcare provider and exploring interventions such as dietary adjustments, probiotics, or emerging therapies (Arthritis Foundation: Gut Health). Early consultation enables tailored strategies and timely intervention, empowering patients to take control of both their gut and joint health for better long-term outcomes.
Disclaimer
The information provided in this article is for general informational purposes only. While we strive to keep the information up-to-date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability, or availability with respect to the article or the information, products, services, or related graphics contained in the article for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
In no event will we be liable for any loss or damage including without limitation, indirect or consequential loss or damage, or any loss or damage whatsoever arising from loss of data or profits arising out of, or in connection with, the use of this article.
Through this article you are able to link to other websites which are not under our control. We have no control over the nature, content, and availability of those sites. The inclusion of any links does not necessarily imply a recommendation or endorse the views expressed within them.
Every effort is made to keep the article up and running smoothly. However, we take no responsibility for, and will not be liable for, the article being temporarily unavailable due to technical issues beyond our control.